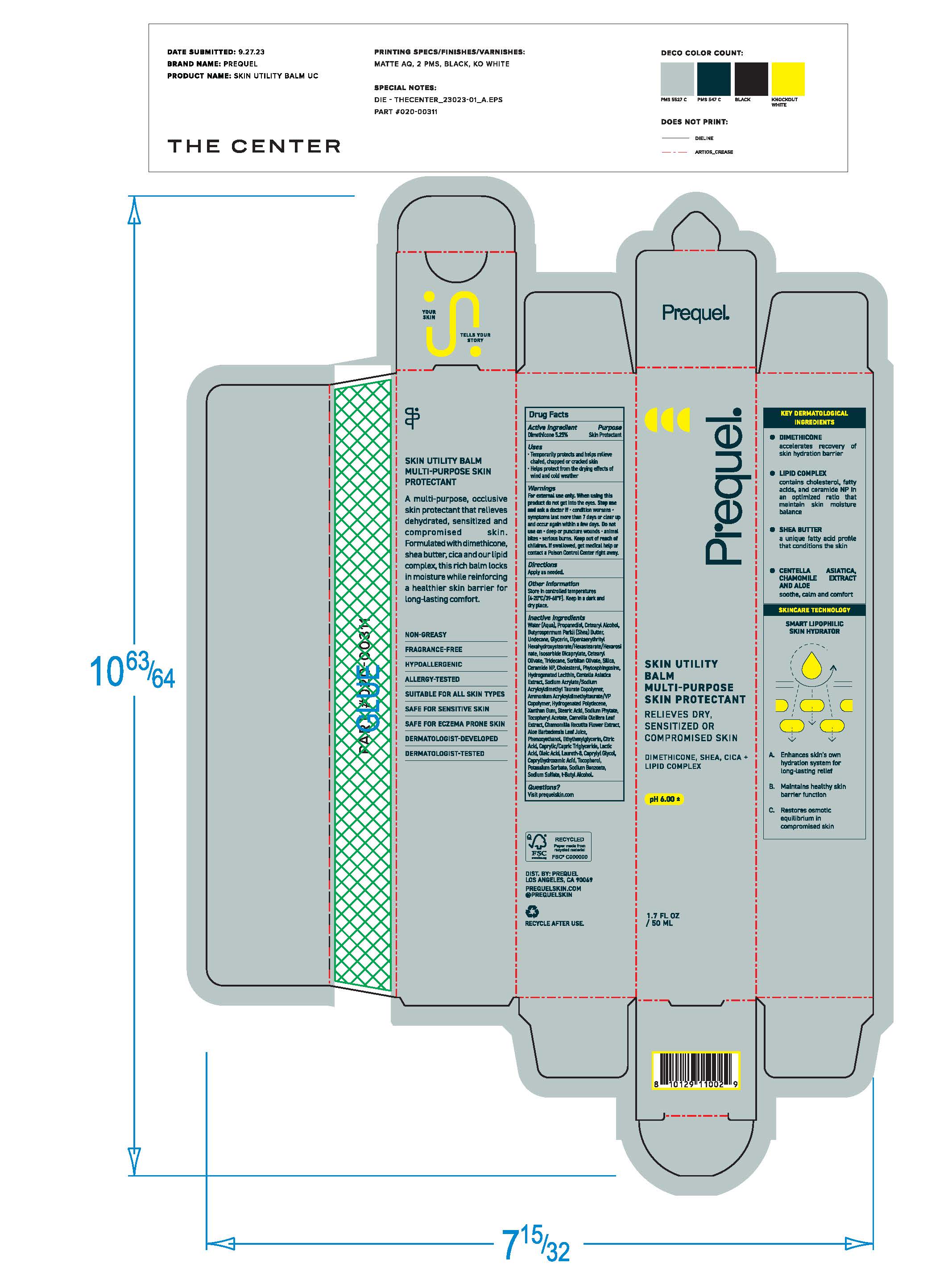
Label: PREQUEL SKIN UTILITY GEL MULTI-PURPOSE SKIN PROTECTANT- skin protectant gel gel
- NDC Code(s): 82800-050-01
- Packager: The Center Brands, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 30, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Purpose
- Active Ingredients
- Uses
- Warnings
- Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
- Directions
-
Inactive ingredients
Water (Aqua), Dimethicone, Panthenol, PEG/PPG/18 Dimethicone Hydroxyethyl Acrylate/Sodium Acryloyldimethyl Tuarate Copolymer, Panthenyl Triacetate, Niacinamide, Naringenin, C15-23 Alkane, 1,2 Hexanediol, C13-14 Alkcane, Phenoxyethanol, Decyl Glucoside, Tetrasodium Glutamate Diacetate, Citric Acid, Sodium Hydroxide.
- Other Information
- Questions?
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
PREQUEL SKIN UTILITY GEL MULTI-PURPOSE SKIN PROTECTANT
skin protectant gel gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82800-050 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GLYCERIN (UNII: PDC6A3C0OX) (GLYCERIN - UNII:PDC6A3C0OX) GLYCERIN 20 mg in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIMETHICONE (UNII: 92RU3N3Y1O) PANTHENOL (UNII: WV9CM0O67Z) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER (100000 MPA.S AT 1.5%) (UNII: 86FQE96TZ4) PANTHENYL ETHYL ETHER ACETATE (UNII: S2FA5ZM21C) NIACINAMIDE (UNII: 25X51I8RD4) NARGENICIN (UNII: XP2QP458K4) C15-23 ALKANE (UNII: J3N6X3YK96) PHENOXYETHANOL (UNII: HIE492ZZ3T) DECYL GLUCOSIDE (UNII: Z17H97EA6Y) TETRASODIUM GLUTAMATE DIACETATE (UNII: 5EHL50I4MY) CITRIC ACID ACETATE (UNII: DSO12WL7AU) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82800-050-01 1 in 1 PACKAGE 10/30/2023 1 50 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 10/30/2023 Labeler - The Center Brands, LLC (076228814)