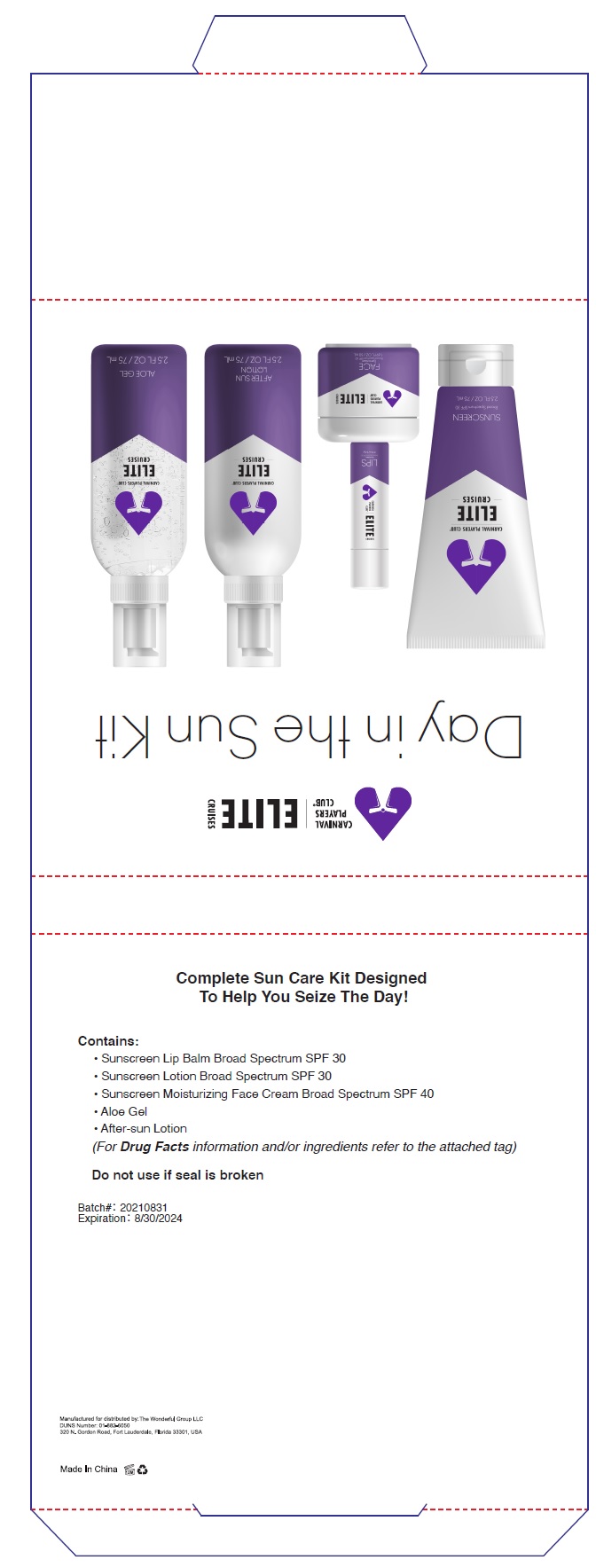
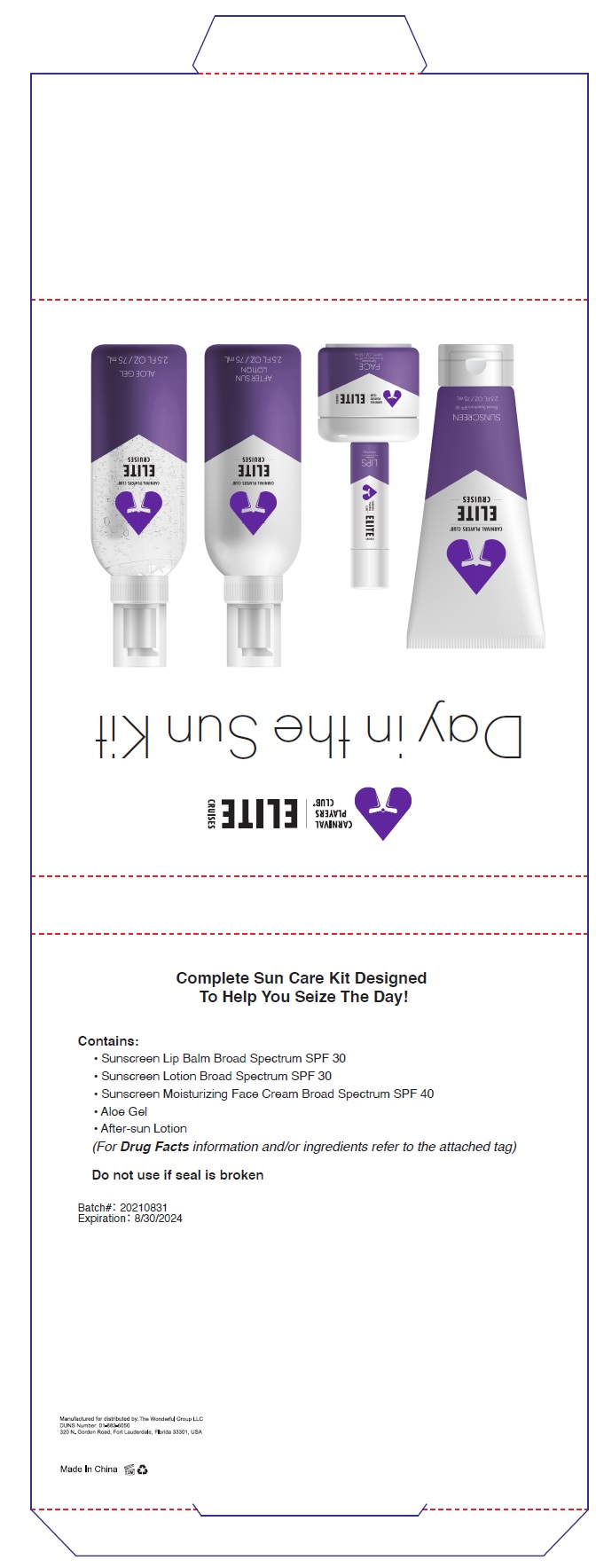
Label: DAY IN THE SUN KIT- avobenzone,homosalate,octisalate,octocrylene kit
- NDC Code(s): 80782-001-01, 80782-002-01, 80782-003-01, 80782-004-01
- Packager: THE WONDERFUL GROUP LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 24, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts for Sunscreen Lotion Broad Spectrum SPF 30
- Active ingredients
- Purpose
- Uses
-
Warnings
For external use only
Do not use on damaged or broken skin
When using this product, keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if irritation or rash develops
Keep out of reach of children. If product is swallowed, get medical attention or contact Poison Control Center immediately. -
Directions
apply liberally and evenly 15 minutes before sun exposure
children under 6 months of age: Ask a doctor
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging.
To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:- limit time in the sun, especially from 10 a.m. - 2 p.m.
- wear long-sleeved shirts, pants, hats, and sunglasses
- reapply at least every 2 hours
- use a water-resistant sunscreen if swimming or sweating
- Other information
-
Inactive ingredients
Water, Butylene Glycol Dicaprylate/Dicaprate, Butyloctyl Salicylate, Propylene Glycol, PEG-100 Stearate,VP/Eicosene Copolymer, Acrylates Copolymer, Steareth-20,Glyceryl Stearate, Steareth-2, Fragrance, Phenoxyethanol,Ethylhexylglycerin, Acrylates/C1 0-30 Alkyl Acrylate
Crosspolymer, Disodium EDTA, Sodium Hyaluronate,Sodium Hydroxide,fragrance - Drug Facts for Sunscreen Moisturizing Face Cream Broad Spectrum SPF 40
- Active ingredients
- Purpose
- Uses
-
Warnings
For external use only
Do not use on damaged or broken skin
When using this product, keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if irritation or rash develops
Keep out of reach of children. If a product is swallowed, get medical attention or contact a Poison Control Center immediately. -
Directions
apply liberally and evenly 15 minutes before sun exposure
children under 6 months of age: Ask a doctor
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging.
To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:- Limit time in the sun, especially from 10 a.m. - 2 p.m.
- Wear long-sleeved shirts, pants, hats, and sunglasses
- reapply at least every 2 hours
- use a water-resistant sunscreen if swimming or sweating
- Other information
-
Inactive ingredients
Water, Vilis Vinifara Seed Oil, Butyloctyl Salicylate, Cocos Nucifera Oil, Glyceryl Stearate, Butylene Glycol Dicaprylate/Dicaprate, Aloe Barbadensis Leaf Extract, VP/Eicosene Copolymer, Acrylates Copolymer, Cetearyl Alcohol,Steareth-20, Steareth-2, Fragrance, Trehalose, Sodium PCA,Prunus Persica(Peach)Resin ExTract, DendrobiumCandidum Stem Extract, Tremella Fuciformis Sporocarp Extract, Cistanche Deserticola Extract, Phenoxyethanol,Ethylhexylglycerin, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Sodium Hyaluronate,TocopheryiAcetate,Sodium Hydroxide,fragrance
- Drug Facts for Sunscreen Lip Balm Broad Spectrum SPF 30
- Active ingredients
- Purpose
- Uses
-
Warnings
For external use only
Do not use on damaged or broken skin
When using this product, keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if irritation or rash develops
Keep out of reach of children. If product is swallowed, get medical attention or contact Poison Control Center immediately. -
Directions
apply liberally and evenly 15 minutes before sun exposure
children under 6 months of age: Ask a doctor
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging.
To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:- limit time in the sun, especially from 10 a.m. - 2 p.m.
- wear long-sleeved shirts, pants, hats, and sunglasses
- reapply at least every 2 hours
- use a water-resistant sunscreen if swimming or sweating
- Other information
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DAY IN THE SUN KIT
avobenzone,homosalate,octisalate,octocrylene kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:80782-001 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:80782-001-01 1 in 1 KIT; Type 1: Convenience Kit of Co-Package 08/01/2021 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 TUBE 75 mL Part 2 1 JAR 50 mL Part 3 1 TUBE 4.5 g Part 4 1 BOTTLE 75 mL Part 5 1 BOTTLE 75 mL Part 1 of 5 SUNSCREEN BROAD SPECTRUM SPF 30
avobenzone,homosalate,octisalate,octocrylene lotionProduct Information Item Code (Source) NDC:80782-002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 8 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 8 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: 75D21FL1PI) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PEG-100 STEARATE (UNII: YD01N1999R) VINYLPYRROLIDONE/EICOSENE COPOLYMER (UNII: 035MV9S1C3) BUTYL ACRYLATE/METHYL METHACRYLATE/METHACRYLIC ACID COPOLYMER (18000 MW) (UNII: JZ1374NL9E) STEARETH-20 (UNII: L0Q8IK9E08) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) STEARETH-2 (UNII: V56DFE46J5) PHENOXYETHANOL (UNII: HIE492ZZ3T) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) HYALURONATE SODIUM (UNII: YSE9PPT4TH) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:80782-002-01 75 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 08/01/2021 Part 2 of 5 SUNSCREEN MOISTURIZING FACE BROAD SPECTRUM SPF 40
avobenzone,homosalate,octisalate,octocrylene creamProduct Information Item Code (Source) NDC:80782-003 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 9 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 9 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GRAPE SEED OIL (UNII: 930MLC8XGG) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) COCONUT OIL (UNII: Q9L0O73W7L) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) ALOE VERA LEAF (UNII: ZY81Z83H0X) VINYLPYRROLIDONE/EICOSENE COPOLYMER (UNII: 035MV9S1C3) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) TREHALOSE (UNII: B8WCK70T7I) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) PRUNUS PERSICA TWIG (UNII: TM1ZG9NCY1) DENDROBIUM MONILIFORME WHOLE (UNII: N7TLA49N8Q) TREMELLA FUCIFORMIS FRUITING BODY (UNII: GG8N28393G) CISTANCHE DESERTICOLA STEM (UNII: 45BEI4ZF64) PHENOXYETHANOL (UNII: HIE492ZZ3T) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) HYALURONATE SODIUM (UNII: YSE9PPT4TH) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: 75D21FL1PI) BUTYL ACRYLATE/METHYL METHACRYLATE/METHACRYLIC ACID COPOLYMER (18000 MW) (UNII: JZ1374NL9E) STEARETH-20 (UNII: L0Q8IK9E08) STEARETH-2 (UNII: V56DFE46J5) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:80782-003-01 50 mL in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 08/01/2021 Part 3 of 5 SUNSCREEN LIP BALM BROAD SPECTRUM SPF 30
avobenzone,homosalate,octisalate,octocrylene gelProduct Information Item Code (Source) NDC:80782-004 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 g HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 9 g in 100 g OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 g OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 9 g in 100 g Inactive Ingredients Ingredient Name Strength ETHYLHEXYL PALMITATE (UNII: 2865993309) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) SODIUM DICHLOROACETATE (UNII: 42932X67B5) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) CANOLA OIL (UNII: 331KBJ17RK) HYDROGENATED SOYBEAN OIL (UNII: A2M91M918C) OLIVE OIL (UNII: 6UYK2W1W1E) BUTYROSPERMUM PARKII (SHEA) BUTTER UNSAPONIFIABLES (UNII: 0C9AC7D6XU) VINYLPYRROLIDONE/EICOSENE COPOLYMER (UNII: 035MV9S1C3) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:80782-004-01 4.5 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 08/01/2021 Part 4 of 5 ALOE GEL
other skin care preparationsProduct Information Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 75 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Cosmetic Part 5 of 5 AFTERSUN LOTION
other skin care preparationsProduct Information Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 75 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Cosmetic Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 08/01/2021 Labeler - THE WONDERFUL GROUP LLC (018826050)