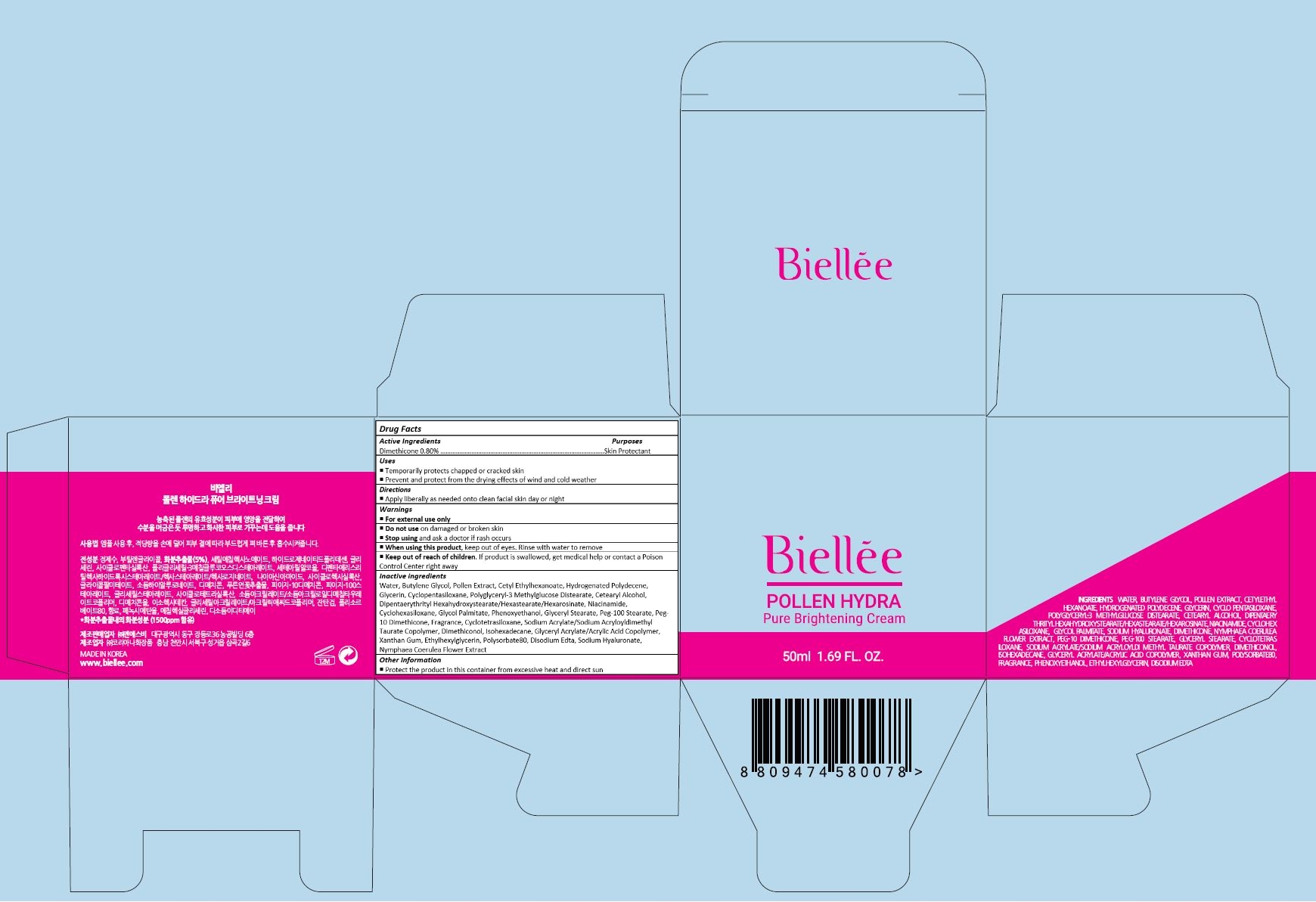
Label: BIELLEE POLLEN HYDRA PURE BRIGHTENING CREAM- dimethicone cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 70784-004-01, 70784-004-02 - Packager: NSB CO., LTD.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated June 21, 2016
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- DOSAGE & ADMINISTRATION
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
-
INACTIVE INGREDIENT
WATER
BUTYLENE GLYCOL
POLLEN EXTRACT
CETYL ETHYLHEXANOATE
HYDROGENATED POLYDECENE
GLYCERIN
CYCLOPENTASILOXANE
POLYGLYCERYL-3 METHYLGLUCOSE DISTEARATE
CETEARYL ALCOHOL
DIPENTAERYTHRITYL HEXAHYDROXYSTEARATE/HEXASTEARATE/HEXAROSINATE
NIACINAMIDE
CYCLOHEXASILOXANE
GLYCOL PALMITATE
PHENOXYETHANOL
GLYCERYL STEARATE
PEG-100 STEARATE
PEG-10 DIMETHICONE
FRAGRANCE
CYCLOTETRASILOXANE
SODIUM ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER
DIMETHICONOL
ISOHEXADECANE
GLYCERYL ACRYLATE/ACRYLIC ACID COPOLYMER
XANTHAN GUM
ETHYLHEXYLGLYCERIN
POLYSORBATE80
DISODIUM EDTA
SODIUM HYALURONATE
NYMPHAEA COERULEA FLOWER EXTRACT - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BIELLEE POLLEN HYDRA PURE BRIGHTENING CREAM
dimethicone creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70784-004 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIMETHICONE (UNII: 92RU3N3Y1O) (DIMETHICONE - UNII:92RU3N3Y1O) DIMETHICONE 0.4 mg in 50 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) BEE POLLEN (UNII: 3729L8MA2C) CETYL ETHYLHEXANOATE (UNII: 134647WMX4) HYDROGENATED POLYDECENE (550 MW) (UNII: U333RI6EB7) GLYCERIN (UNII: PDC6A3C0OX) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) NIACINAMIDE (UNII: 25X51I8RD4) CYCLOMETHICONE 6 (UNII: XHK3U310BA) GLYCOL PALMITATE (UNII: T80A3T38WE) PHENOXYETHANOL (UNII: HIE492ZZ3T) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) PEG-100 STEARATE (UNII: YD01N1999R) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) CYCLOMETHICONE 4 (UNII: CZ227117JE) SODIUM ACRYLATE/SODIUM ACRYLOYLDIMETHYLTAURATE COPOLYMER (4000000 MW) (UNII: 1DXE3F3OZX) DIMETHICONOL (2000 CST) (UNII: T74O12AN6Y) ISOHEXADECANE (UNII: 918X1OUF1E) XANTHAN GUM (UNII: TTV12P4NEE) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) POLYSORBATE 80 (UNII: 6OZP39ZG8H) EDETATE DISODIUM (UNII: 7FLD91C86K) HYALURONATE SODIUM (UNII: YSE9PPT4TH) NYMPHAEA CAERULEA FLOWER (UNII: S9560USZ74) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70784-004-02 1 in 1 CARTON 06/21/2016 1 NDC:70784-004-01 50 mL in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 06/21/2016 Labeler - NSB CO., LTD. (689846922) Registrant - NSB CO., LTD. (689846922) Establishment Name Address ID/FEI Business Operations NSB CO., LTD. 689846922 manufacture(70784-004)