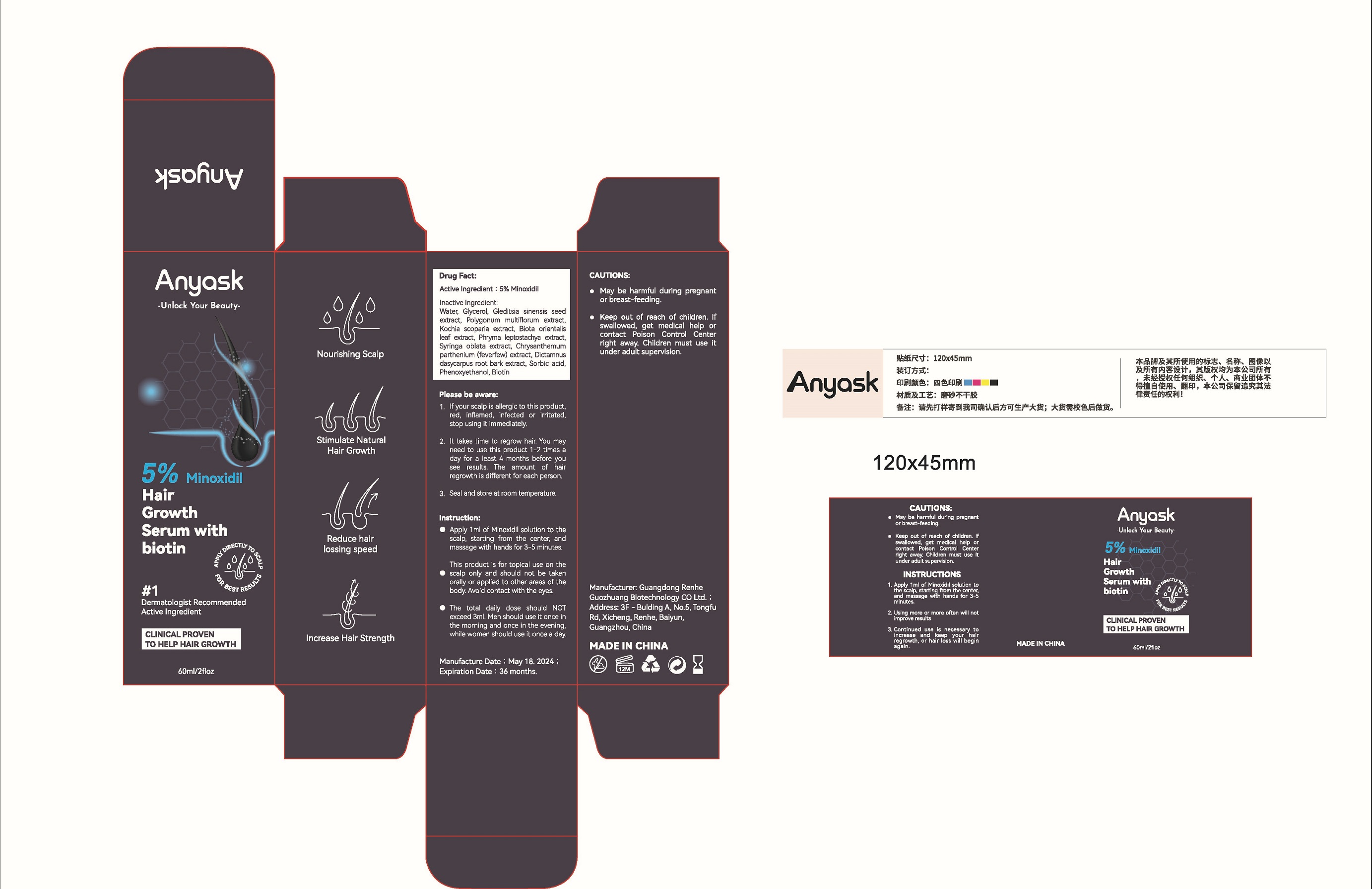
Label: ANYASK 5% MINOXIDIL HAIR GROWTH SERUM WITH BIOTIN- minoxidil liquid
- NDC Code(s): 84450-001-01
- Packager: Shenzhen Dong bu luo Technology Co., LTD
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated June 25, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- PURPOSE
- USE
-
WARNINGS
For external use only
Avoid contact with eyes
When using
This product is for topical use on the scalp only and should not betaken orally or applied to other areas of the body. Avoid contact with the eyes.
- DIRECTIONS
-
INACTIVE INGREDIENTS
Water, Glycerol, Gleditsia sinensis seed extract, Polygonum multiflorum extract, Kochia scoparia extract, Biota orientalis leaf extract, Phryma leptostachya extract, Syringa oblata extract, Chrysanthemum parthenium (feverfew) extract, Dictamnus dasycarpus root bark extract, Sorbic acid, Phenoxyethanol, Biotin
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ANYASK 5% MINOXIDIL HAIR GROWTH SERUM WITH BIOTIN
minoxidil liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84450-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MINOXIDIL (UNII: 5965120SH1) (MINOXIDIL - UNII:5965120SH1) MINOXIDIL 5 g in 100 mL Inactive Ingredients Ingredient Name Strength GLEDITSIA SINENSIS SEED (UNII: T651YZ712C) BIOTIN (UNII: 6SO6U10H04) DICTAMNUS DASYCARPUS ROOT BARK (UNII: LA97176ILS) PHENOXYETHANOL (UNII: HIE492ZZ3T) BASSIA SCOPARIA WHOLE (UNII: 240G38P85Z) SYRINGA OBLATA WHOLE (UNII: T272Z8620J) WATER (UNII: 059QF0KO0R) REYNOUTRIA MULTIFLORA WHOLE (UNII: 85S46HFR8A) PHRYMA LEPTOSTACHYA WHOLE (UNII: 6G287VJM69) GLYCERIN (UNII: PDC6A3C0OX) TANACETUM PARTHENIUM WHOLE (UNII: 6GE7Z0761K) SORBIC ACID (UNII: X045WJ989B) PLATYCLADUS ORIENTALIS LEAF (UNII: 32E5V7G32B) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84450-001-01 60 mL in 1 BOTTLE; Type 0: Not a Combination Product 06/25/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M032 06/25/2024 Labeler - Shenzhen Dong bu luo Technology Co., LTD (614263505) Establishment Name Address ID/FEI Business Operations Shenzhen Dong bu luo Technology Co., LTD 614263505 manufacture(84450-001)