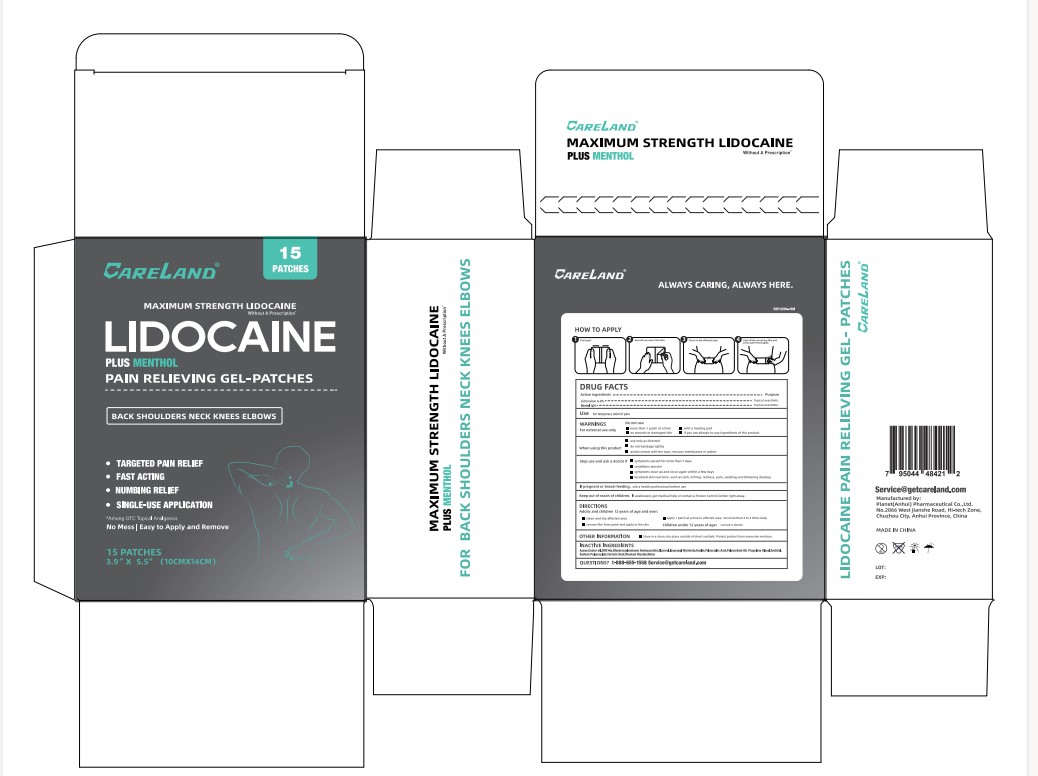
Label: CARELAND LIDOCAINE PLUS MENTHOL PAIN RELIEVING- lidocaine,menthol, unspecified form patch
- NDC Code(s): 75568-016-01
- Packager: Planet (Anhui) Pharmaceutical Co. Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated April 24, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Drug FactsDrug Facts
-
Active IngredientsLidocaine 4.0% Menthol 1.0%
-
PurposeTopical Anesthetic
-
Usefor temperorary relief of pain
-
WarningsFor external use only
-
Do not usemore than 1 patch at a time - on wounds or damaged skin - with a heating pad - if you are allergic to any ingredients of this product
-
when using this productuse only as directed - do not bandage tightly - avoid contact with the eyes, mucous membranes or rashes
-
Stop use and ask a doctor ifsymptoms persist for more than 7 days - conditions worsen - symptoms clear up and occur again within few days - localized skin reactions, such as rash, itching, redness, pain, swelling and blistering ...
-
If pregnant or breasatfeeding,ask a health professional before use
-
Keep out of reach of children.If swallowed, get medical help or contact a Poison Control Center right away.
-
DirectionsAdults and Children 12 years of age and over: clean and dry affected area - remove film from patch and apply to skin - apply 1 patch at a time to affected area, not more than 3 to 4 times ...
-
Other InformationStore in a clean, dry place outside of direct sunlight. Protect product from excessive moisture
-
Inactive IngredientsAzone, Castor oil, CMC-Na, Dihydroxyaluminum Aminoacetate, Glycerol, Isopropyl Myristate, Kaolin, Polyacrylic Acid, Polysorbate 80, Propylene Glycol, Sorbitol, Sodium Polyacrylate, Tartaric Acid ...
-
Questions?1-888-655-1558 Service@getcareland.com
-
Careland Lidocaine Plus Menthol Pain Relieving Gel Patches Label
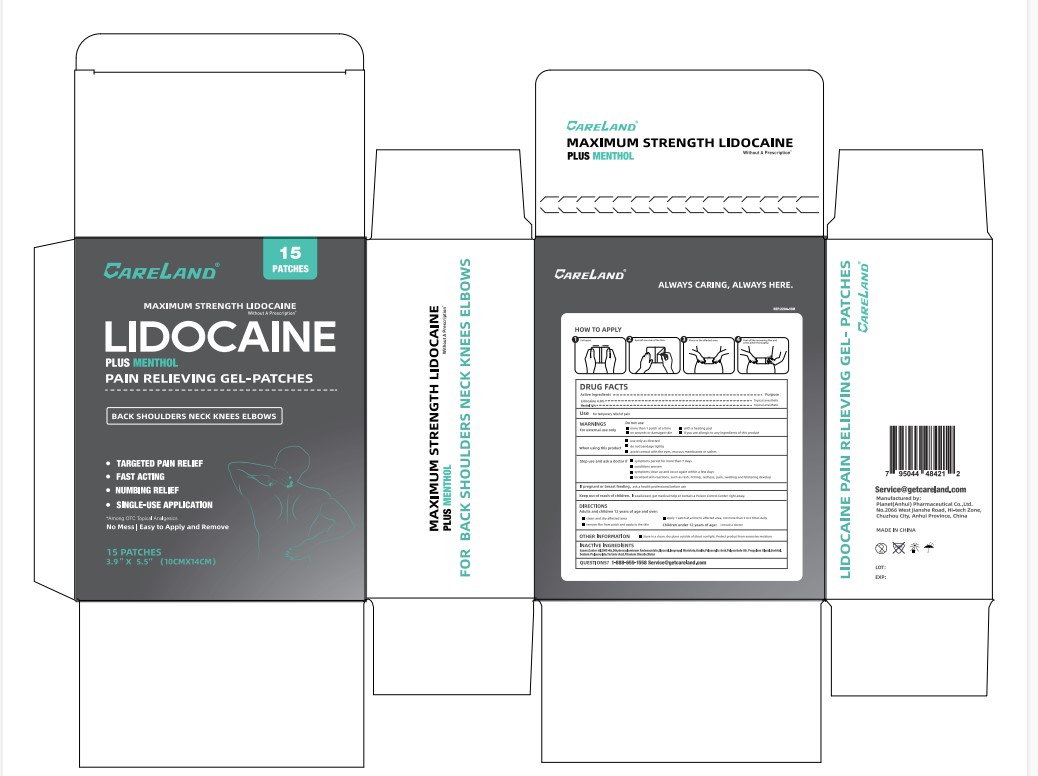
-
INGREDIENTS AND APPEARANCEProduct Information