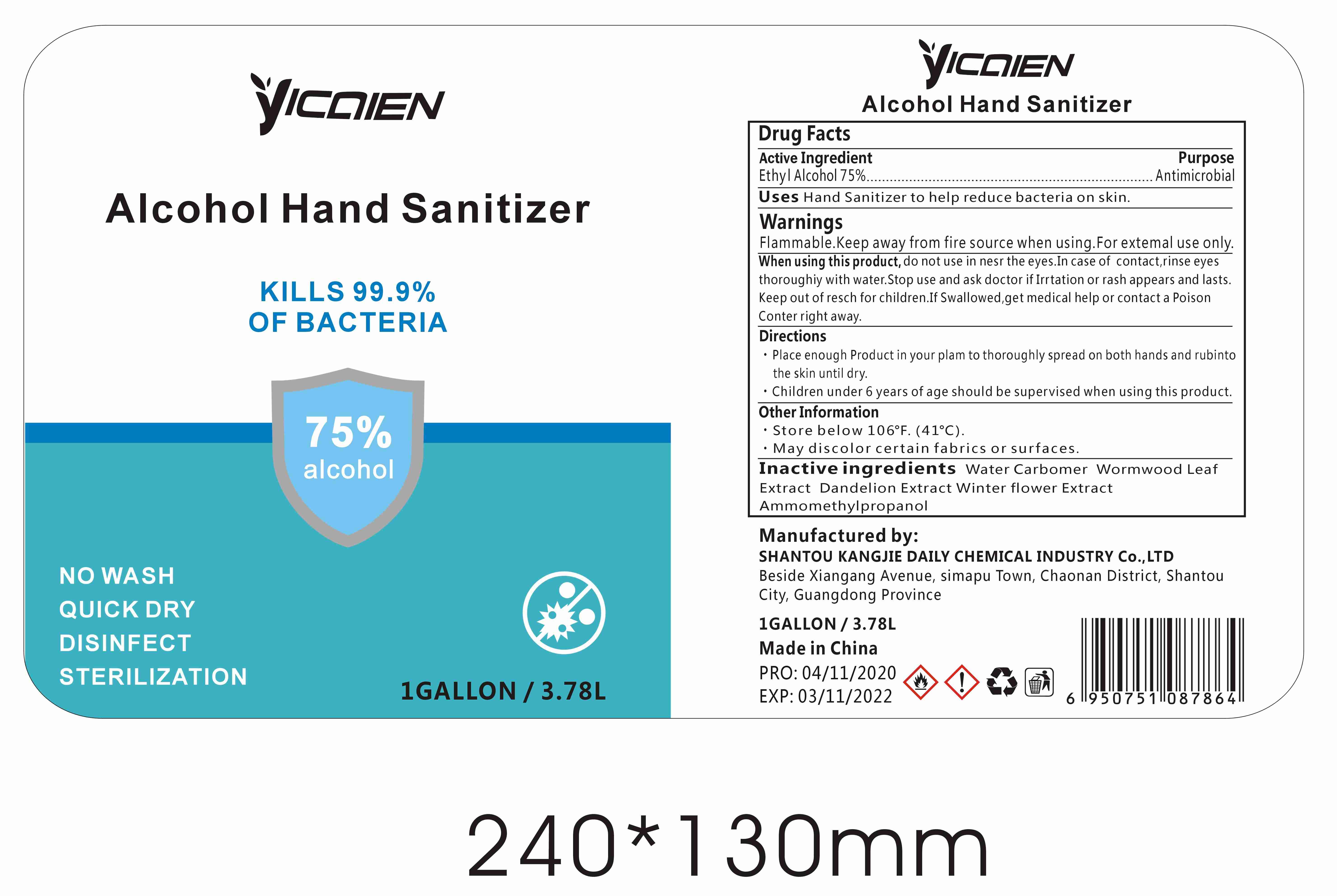
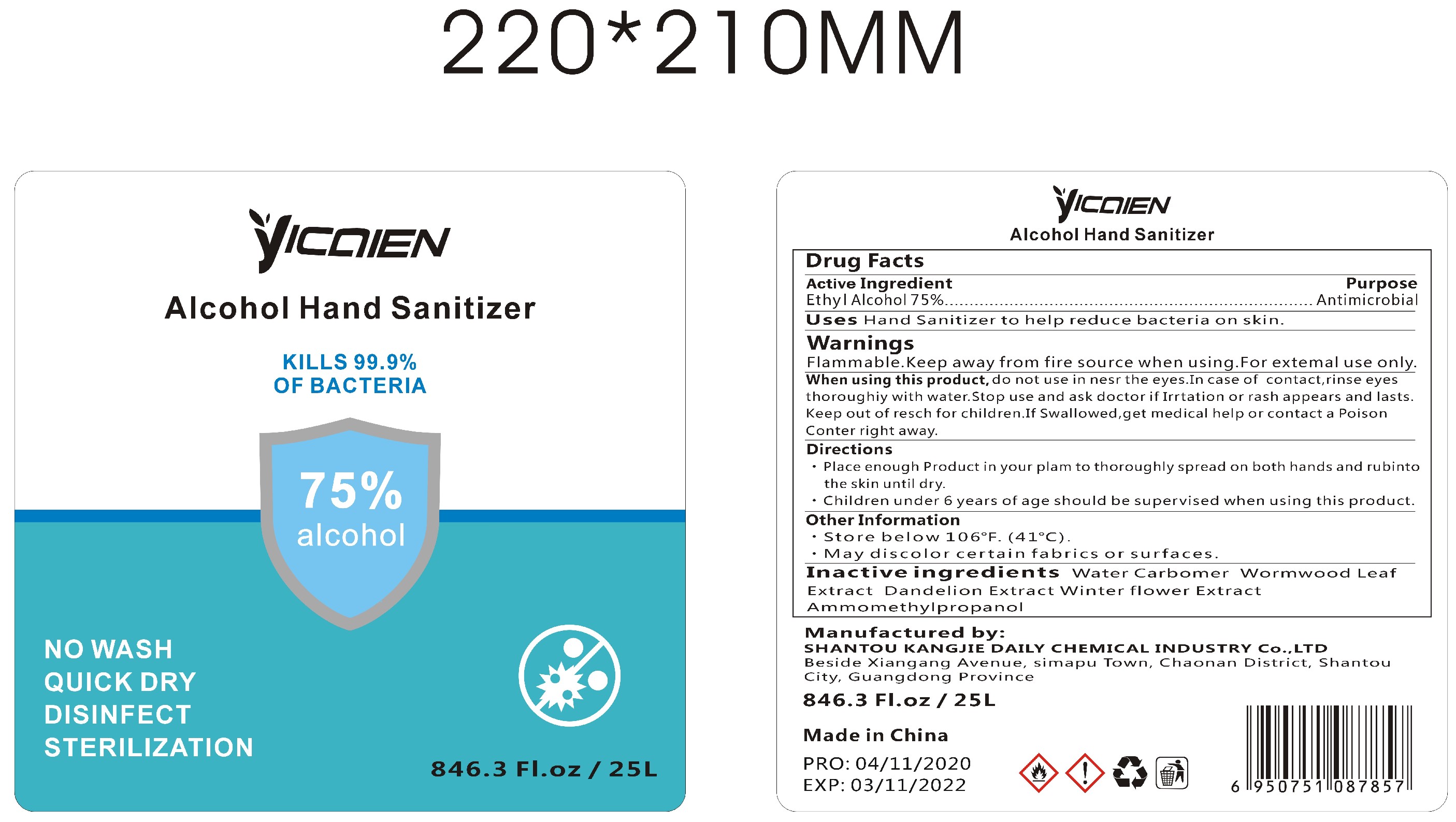
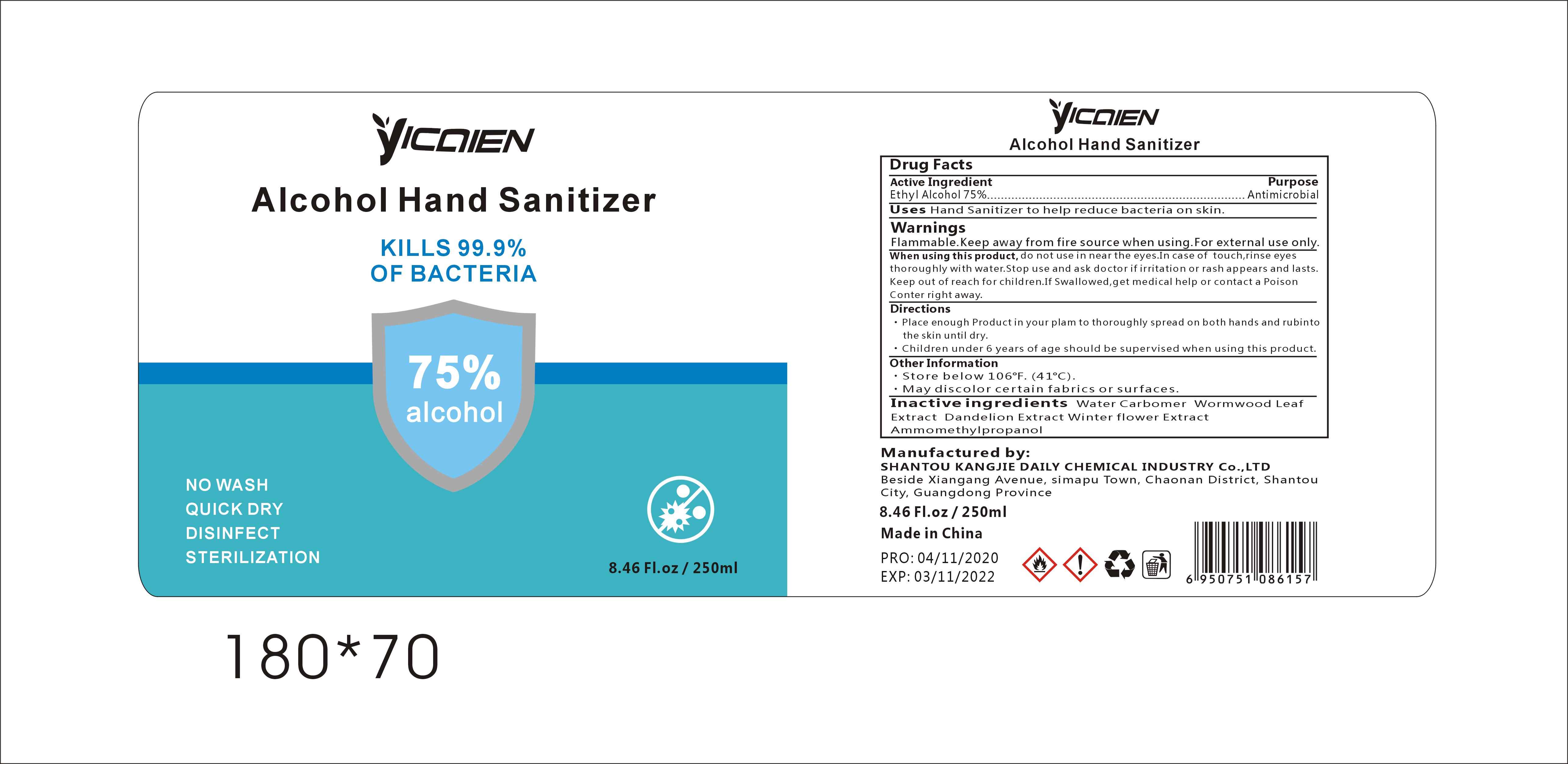
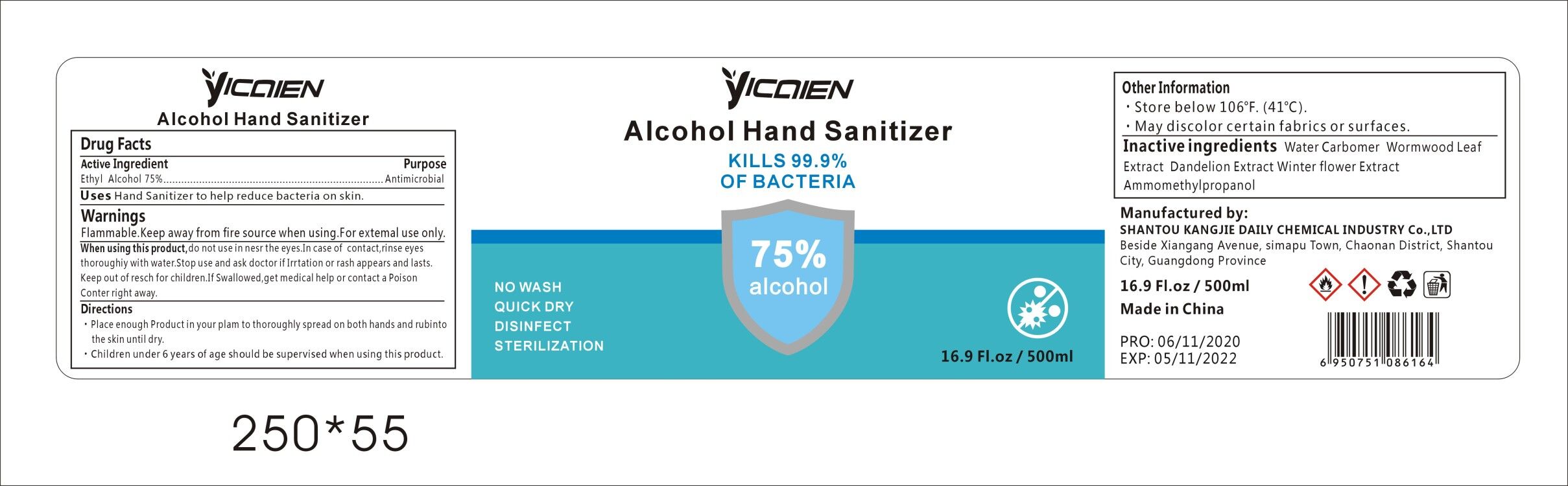
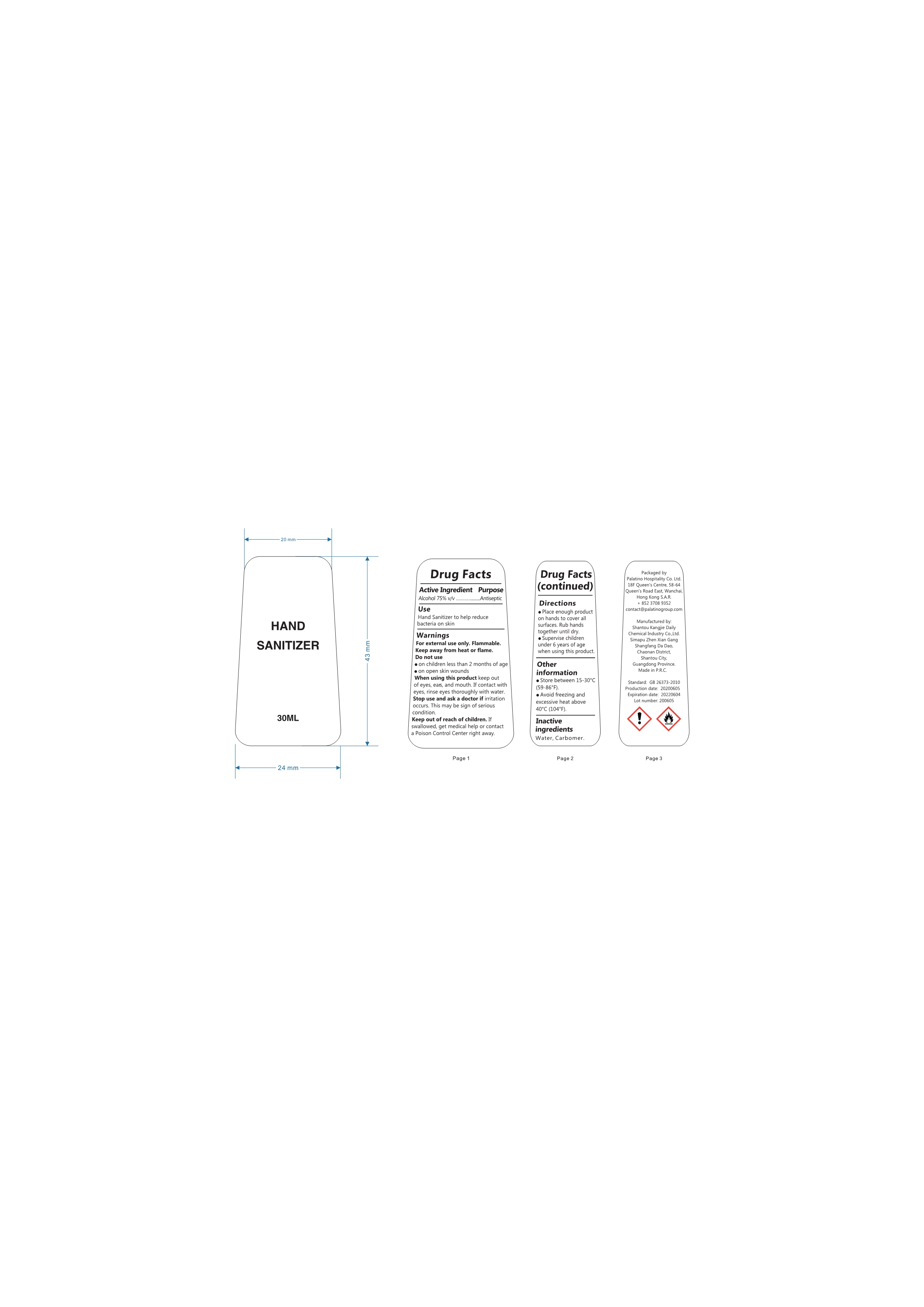
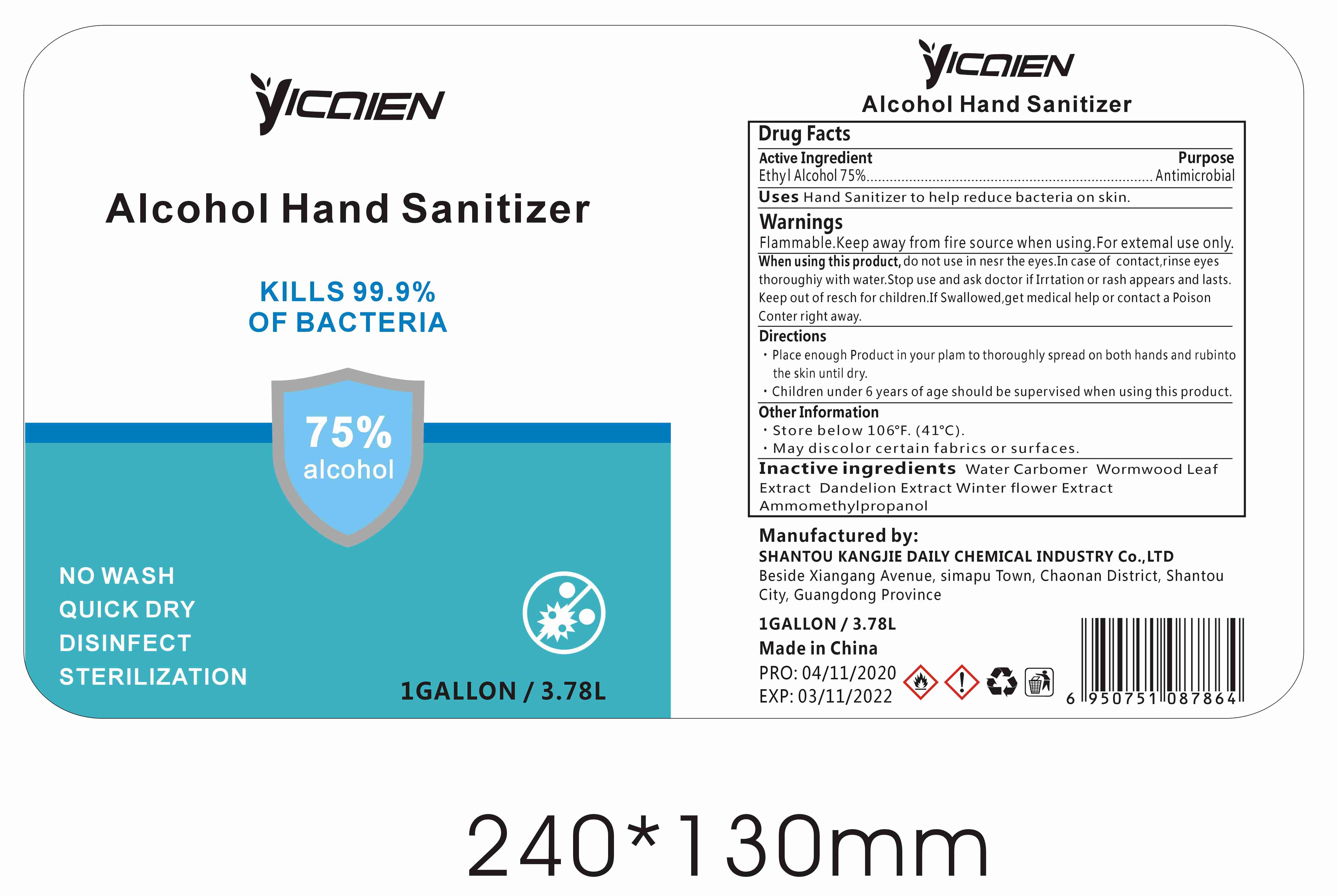
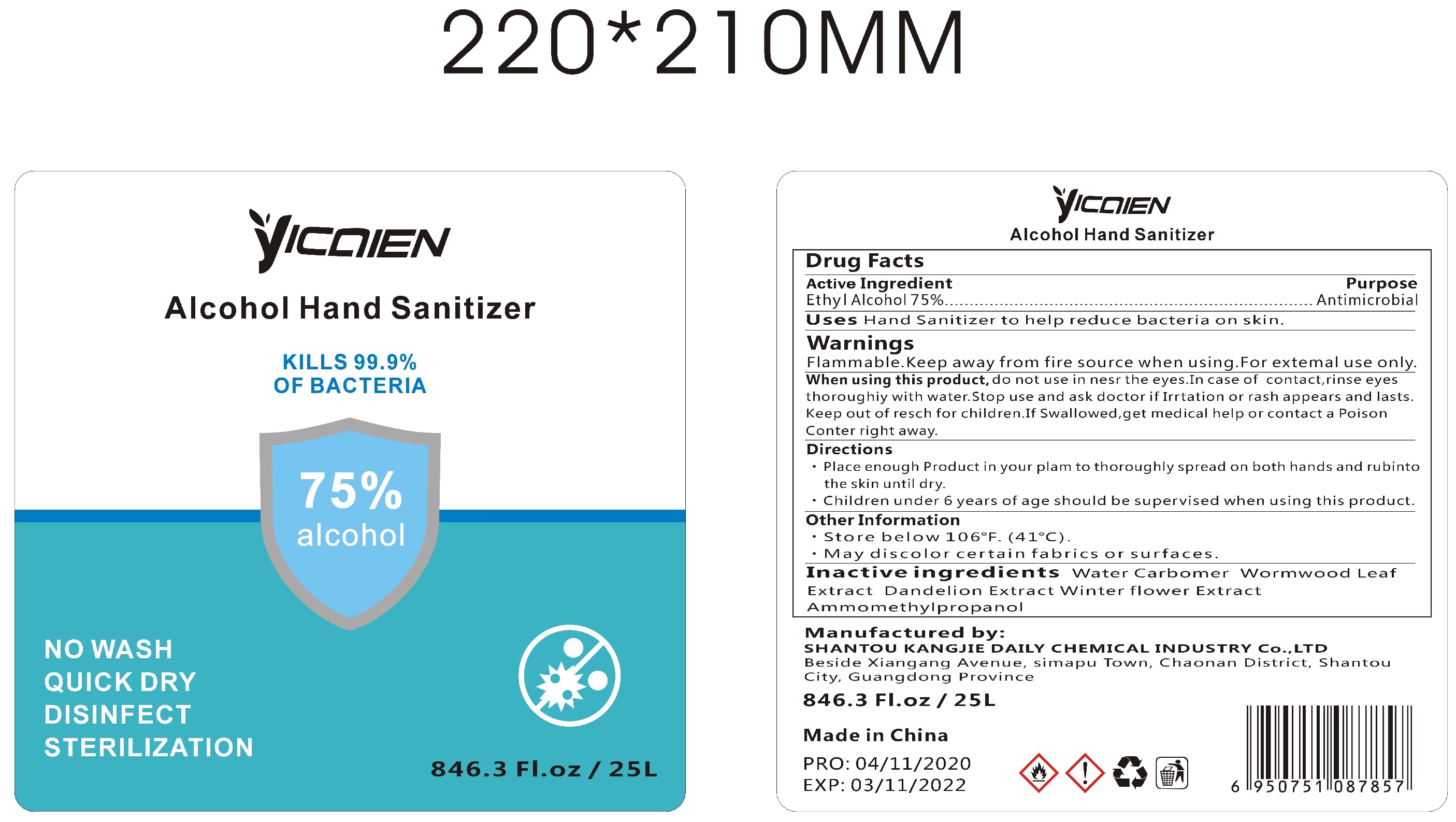
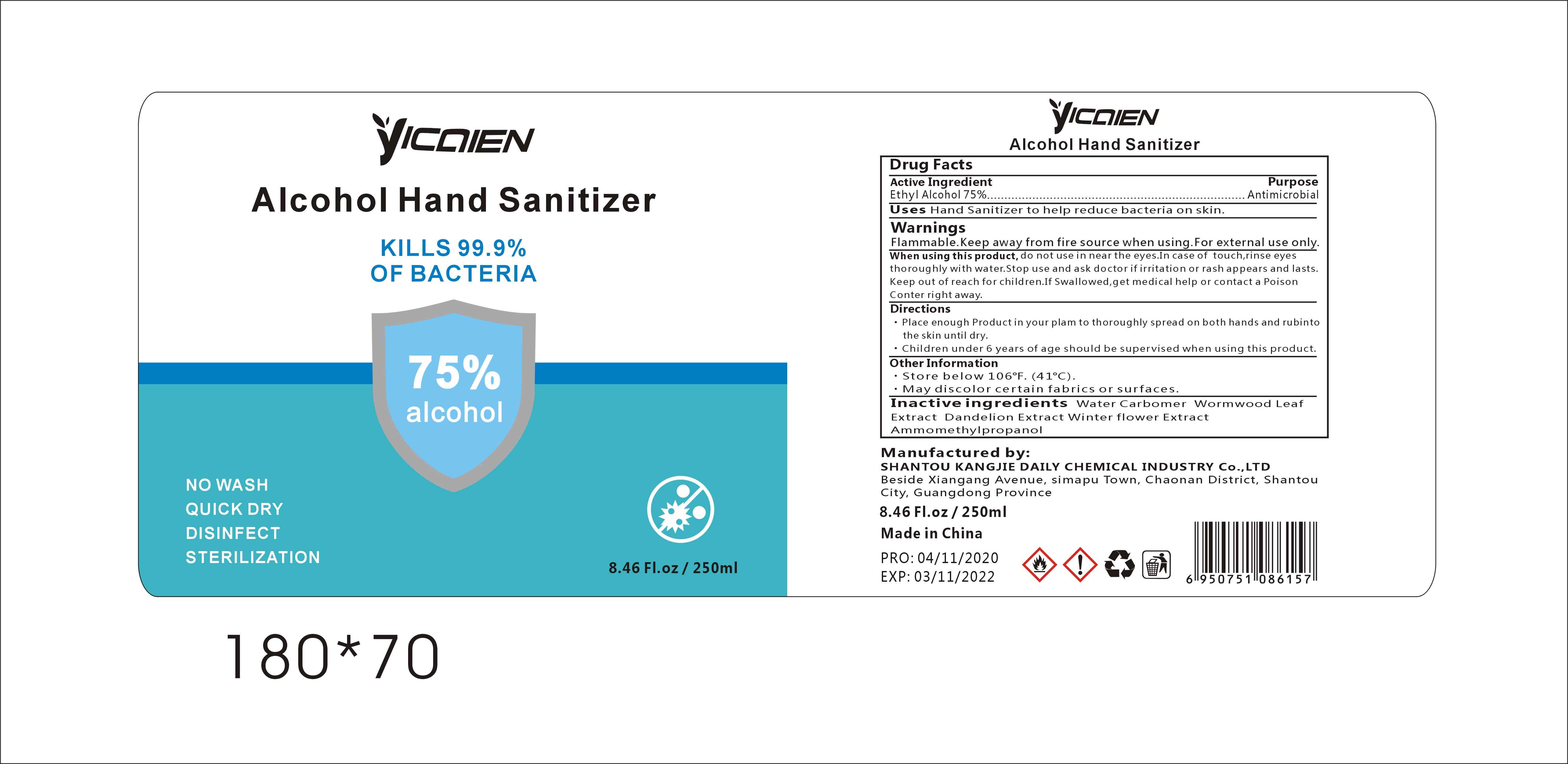
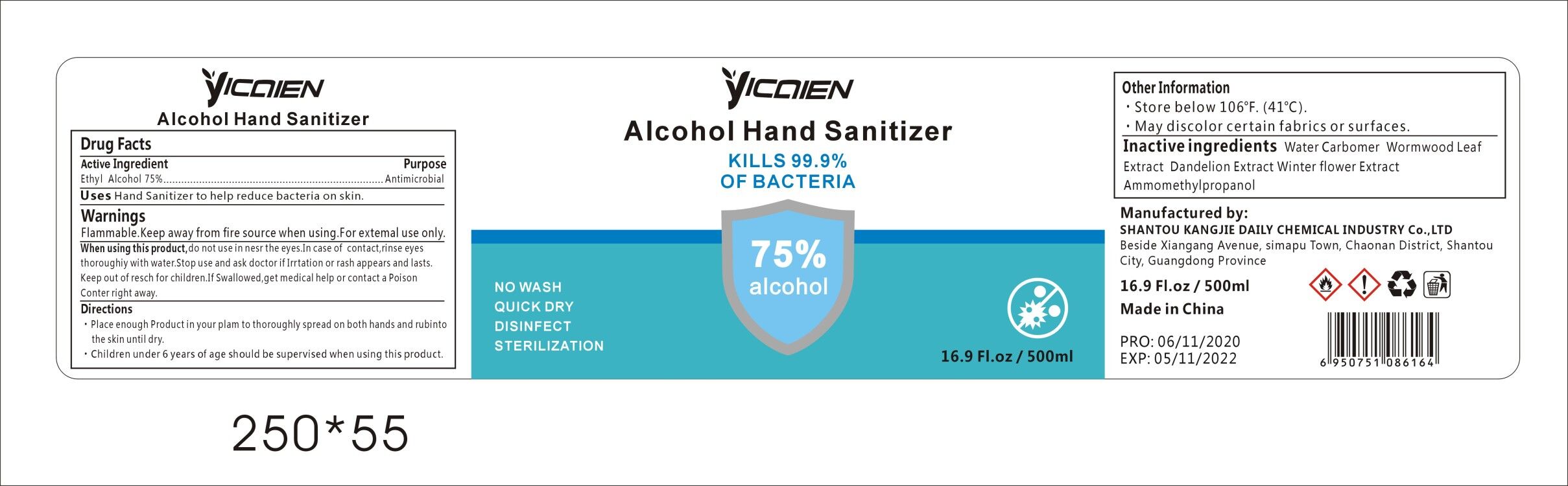
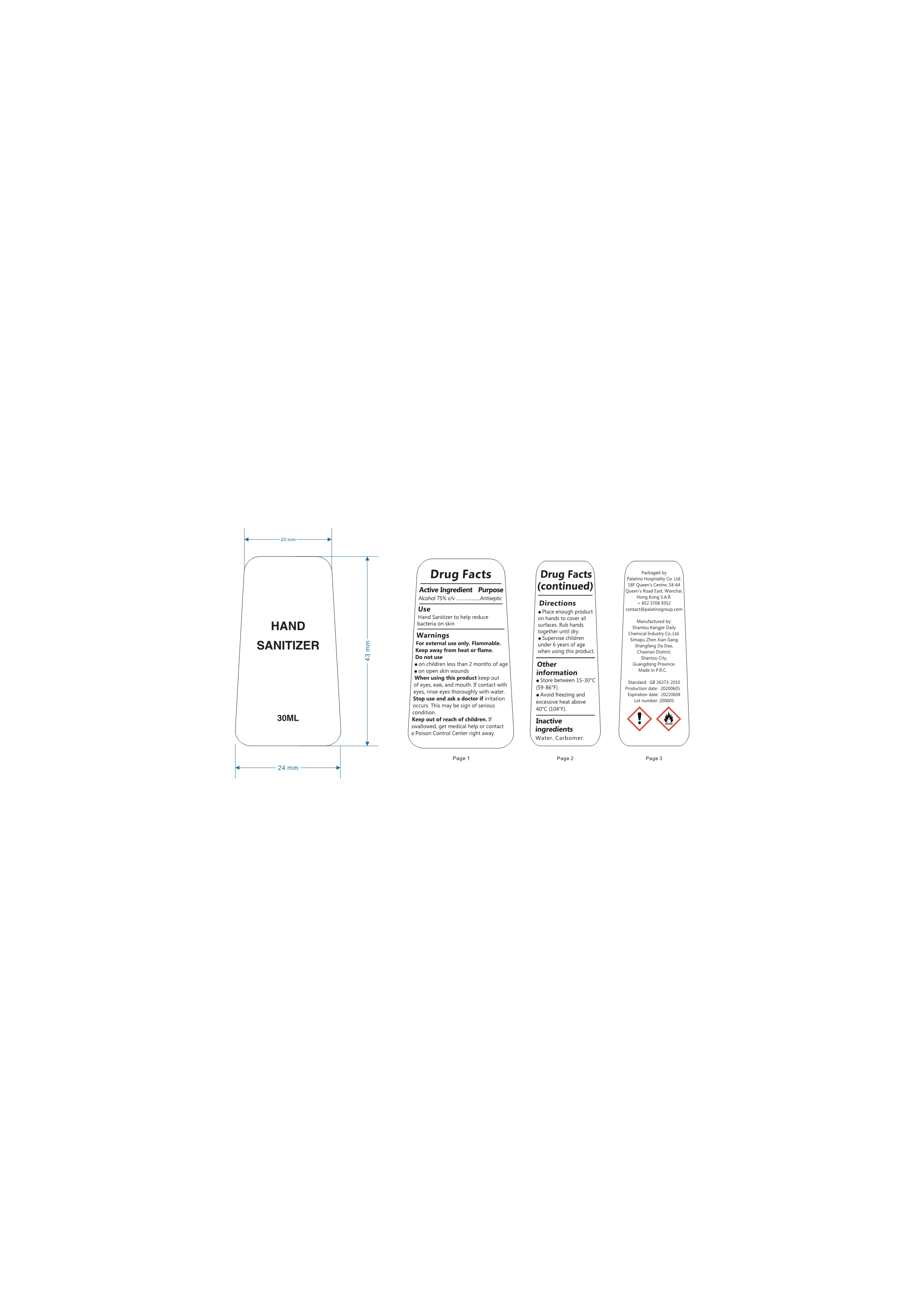
Label: ALCOHOL HAND SANITIZER gel
-
NDC Code(s):
74047-002-01,
74047-002-02,
74047-002-03,
74047-002-04, view more74047-002-05, 74047-002-06, 74047-002-07, 74047-002-08, 74047-002-09, 74047-002-10, 74047-002-11, 74047-002-12, 74047-002-13, 74047-002-14, 74047-002-15, 74047-002-16, 74047-002-17, 74047-002-18
- Packager: Shantou Kangjie Daily Chemical Industry Co.,Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 11, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Uses
-
WARNINGS
Flammable.Keep away from fire or flame.For external use only.When using this product,do not use in or near the eyes.In case of contact,rinse eyes thoroughly with water.Stop use and ask doctor if irritation or rash appears and lasts.Keep out of reach for children. If swallowed, get medical help or contact a Poison Control Center right away.
- DO NOT USE
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Other Information
- INACTIVE INGREDIENTS
- Package Label - Principal Display Panel
-
INGREDIENTS AND APPEARANCE
ALCOHOL HAND SANITIZER
alcohol hand sanitizer gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:74047-002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 75 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CARBOMER COPOLYMER TYPE A (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 71DD5V995L) ARTEMISIA ARGYI LEAF (UNII: 2JYC99Q0WZ) BLACKBACK FOUNDER (UNII: PX3N99X6C9) AMINOMETHYLPROPANOL (UNII: LU49E6626Q) TARAXACUM OFFICINALE ROOT (UNII: 9DE5YCO0RU) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:74047-002-01 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/16/2020 2 NDC:74047-002-06 250 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/16/2020 3 NDC:74047-002-07 500 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/16/2020 4 NDC:74047-002-08 1000 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/16/2020 5 NDC:74047-002-02 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/16/2020 6 NDC:74047-002-03 50 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/16/2020 7 NDC:74047-002-04 100 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/16/2020 8 NDC:74047-002-05 236 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/16/2020 9 NDC:74047-002-09 5000 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/16/2020 10 NDC:74047-002-10 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/16/2020 11 NDC:74047-002-11 50 mL in 1 TUBE; Type 0: Not a Combination Product 04/16/2020 12 NDC:74047-002-13 100 mL in 1 TUBE; Type 0: Not a Combination Product 04/16/2020 13 NDC:74047-002-14 100 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/16/2020 14 NDC:74047-002-12 60 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/16/2020 15 NDC:74047-002-16 19000 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/16/2020 16 NDC:74047-002-17 20000 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/16/2020 17 NDC:74047-002-18 25000 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/16/2020 18 NDC:74047-002-15 3780 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/16/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 04/16/2020 Labeler - Shantou Kangjie Daily Chemical Industry Co.,Ltd (527128712) Registrant - Shantou Kangjie Daily Chemical Industry Co.,Ltd (527128712) Establishment Name Address ID/FEI Business Operations Shantou Kangjie Daily Chemical Industry Co.,Ltd 527128712 manufacture(74047-002)