Label: DR. ZONSKIN NUNSSUP JARA- glycerin, biotin liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 82934-301-01 - Packager: J 1010 Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated August 25, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
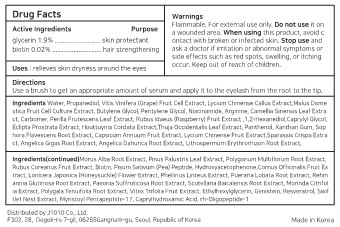
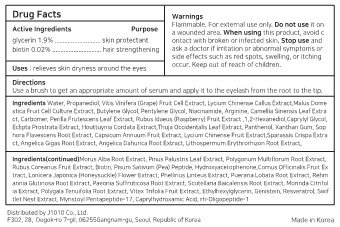
- Active ingredients
- Purpose
- Use
- Warnings
- Warnings
- Warnings
- Warnings
- Warnings
- Directions
-
Inactive ingredients
Water, Propanediol, Vitis Vinifera (Grape) Fruit Cell Extract, Lycium Chinense Callus Extract,Malus Dome stica Fruit Cell Culture Extract, Butylene Glycol, Pentylene Glycol, Niacinamide, Arginine, Camellia Sinensis Leaf Extra ct, Carbomer, Perilla Frutescens Leaf Extract, Rubus Idaeus (Raspberry) Fruit Extract ,1,2-Hexanediol,Caprylyl Glycol, Eclipta Prostrata Extract, Houttuynia Cordata Extract,Thuja Occidentalis Leaf Extract, Panthenol, Xanthan Gum, Sop hora Flavescens Root Extract, Capsicum Annuum Fruit Extract, Lycium Chinense Fruit Extract,Sparassis Crispa Extra ct, Angelica Gigas Root Extract, Angelica Dahurica Root Extract, Lithospermum Erythrorhizon Root Extract,
Morus Alba Root Extract, Pinus Palustris Leaf Extract, Polygonum Multiflorum Root Extract, Rubus Coreanus Fruit Extract, Biotin, Pisum Sativum (Pea) Peptide, Hydroxyacetophenone,Cornus Officinalis Fruit Ex tract, Lonicera Japonica (Honeysuckle) Flower Extract, Phellinus Linteus Extract, Pueraria Lobata Root Extract, Rehm annia Glutinosa Root Extract, Paeonia Suffruticosa Root Extract, Scutellaria Baicalensis Root Extract, Morinda Citrifol ia Extract, Polygala Tenuifolia Root Extract, Vitex Trifolia Fruit Extract, Ethylhexylglycerin, Genistein, Resveratrol, Swif tlet Nest Extract, Myristoyl Pentapeptide-17, Caprylhydroxamic Acid, rh-Oligopeptide-1
- Label
-
INGREDIENTS AND APPEARANCE
DR. ZONSKIN NUNSSUP JARA
glycerin, biotin liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82934-301 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GLYCERIN (UNII: PDC6A3C0OX) (GLYCERIN - UNII:PDC6A3C0OX) GLYCERIN 1.9 g in 100 mL BIOTIN (UNII: 6SO6U10H04) (BIOTIN - UNII:6SO6U10H04) BIOTIN 0.02 g in 100 mL Inactive Ingredients Ingredient Name Strength ACENEURAMIC ACID (UNII: GZP2782OP0) MYRISTOYL PENTAPEPTIDE-4 (UNII: PMA59A699X) PROPANEDIOL (UNII: 5965N8W85T) WINE GRAPE (UNII: 3GOV20705G) APPLE (UNII: B423VGH5S9) LYCIUM CHINENSE WHOLE (UNII: C945L5W38R) WATER (UNII: 059QF0KO0R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) PENTYLENE GLYCOL (UNII: 50C1307PZG) NIACINAMIDE (UNII: 25X51I8RD4) ARGININE (UNII: 94ZLA3W45F) GREEN TEA LEAF (UNII: W2ZU1RY8B0) PERILLA FRUTESCENS LEAF (UNII: T4L5881Y68) RASPBERRY (UNII: 4N14V5R27W) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ECLIPTA PROSTRATA LEAF (UNII: H86R96580E) HOUTTUYNIA CORDATA FLOWERING TOP (UNII: RH041UUZ22) THUJA OCCIDENTALIS LEAF (UNII: 0T0DQN8786) PANTHENOL (UNII: WV9CM0O67Z) XANTHAN GUM (UNII: TTV12P4NEE) SOPHORA FLAVESCENS ROOT (UNII: IYR6K8KQ5K) PAPRIKA (UNII: X72Z47861V) LYCIUM CHINENSE FRUIT (UNII: TG711Q7A1Q) SPARASSIS CRISPA WHOLE (UNII: PC7597U951) ANGELICA GIGAS ROOT (UNII: 32766B2FHX) ANGELICA DAHURICA ROOT (UNII: 1V63N2S972) LITHOSPERMUM ERYTHRORHIZON ROOT (UNII: 9I70D8O47I) MORUS ALBA ROOT (UNII: CST1G9BZGD) PINUS PALUSTRIS LEAF (UNII: OT6R5143A1) REYNOUTRIA MULTIFLORA ROOT (UNII: AUZ3VD75MC) RUBUS COREANUS FRUIT (UNII: 18VM55XVF7) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) PEA (UNII: W4X7H8GYFM) HYDROXYACETOPHENONE (UNII: G1L3HT4CMH) CORNUS OFFICINALIS FRUIT (UNII: 23NL8NQ187) LONICERA JAPONICA FLOWER (UNII: 4465L2WS4Y) PHELLINUS LINTEUS WHOLE (UNII: YVO92B1UCA) PUERARIA MONTANA VAR. LOBATA ROOT (UNII: PET93F4I3C) REHMANNIA GLUTINOSA ROOT (UNII: 1BEM3U6LQQ) PAEONIA X SUFFRUTICOSA ROOT (UNII: 7M7E9A2C8J) SCUTELLARIA BAICALENSIS ROOT (UNII: 7J95K7ID2S) MORINDA CITRIFOLIA LEAF (UNII: 7UOL7P5FF5) POLYGALA TENUIFOLIA ROOT (UNII: 5S7W573MTU) VITEX TRIFOLIA FRUIT (UNII: Q04E1F5351) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) GENISTEIN (UNII: DH2M523P0H) RESVERATROL (UNII: Q369O8926L) CAPRYLHYDROXAMIC ACID (UNII: UPY805K99W) NEPIDERMIN (UNII: TZK30RF92W) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82934-301-01 7 mL in 1 BOTTLE, WITH APPLICATOR; Type 0: Not a Combination Product 08/25/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 08/25/2022 Labeler - J 1010 Co., Ltd. (695562861) Registrant - J 1010 Co., Ltd. (695562861) Establishment Name Address ID/FEI Business Operations J 1010 Co., Ltd. 695562861 manufacture(82934-301)