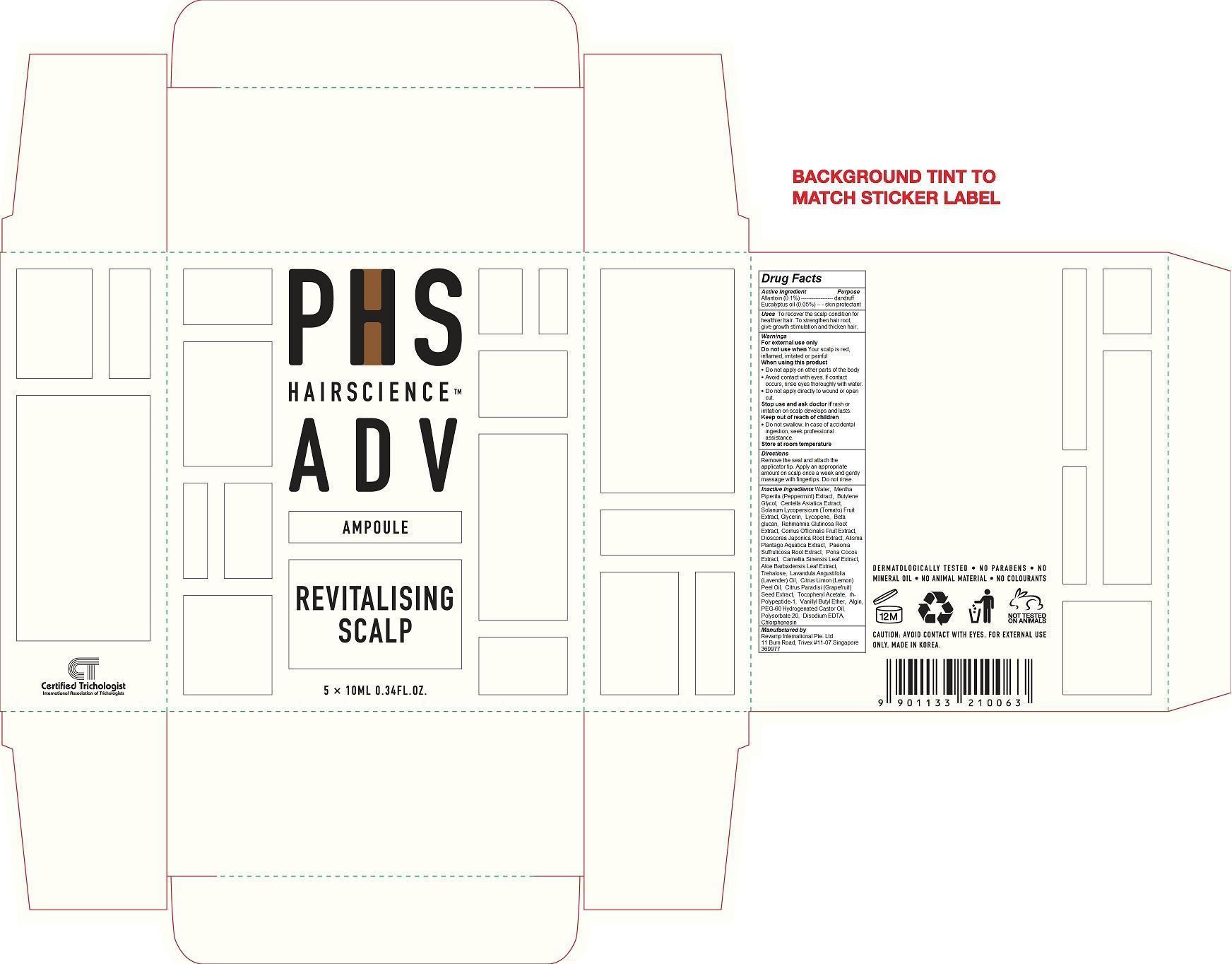
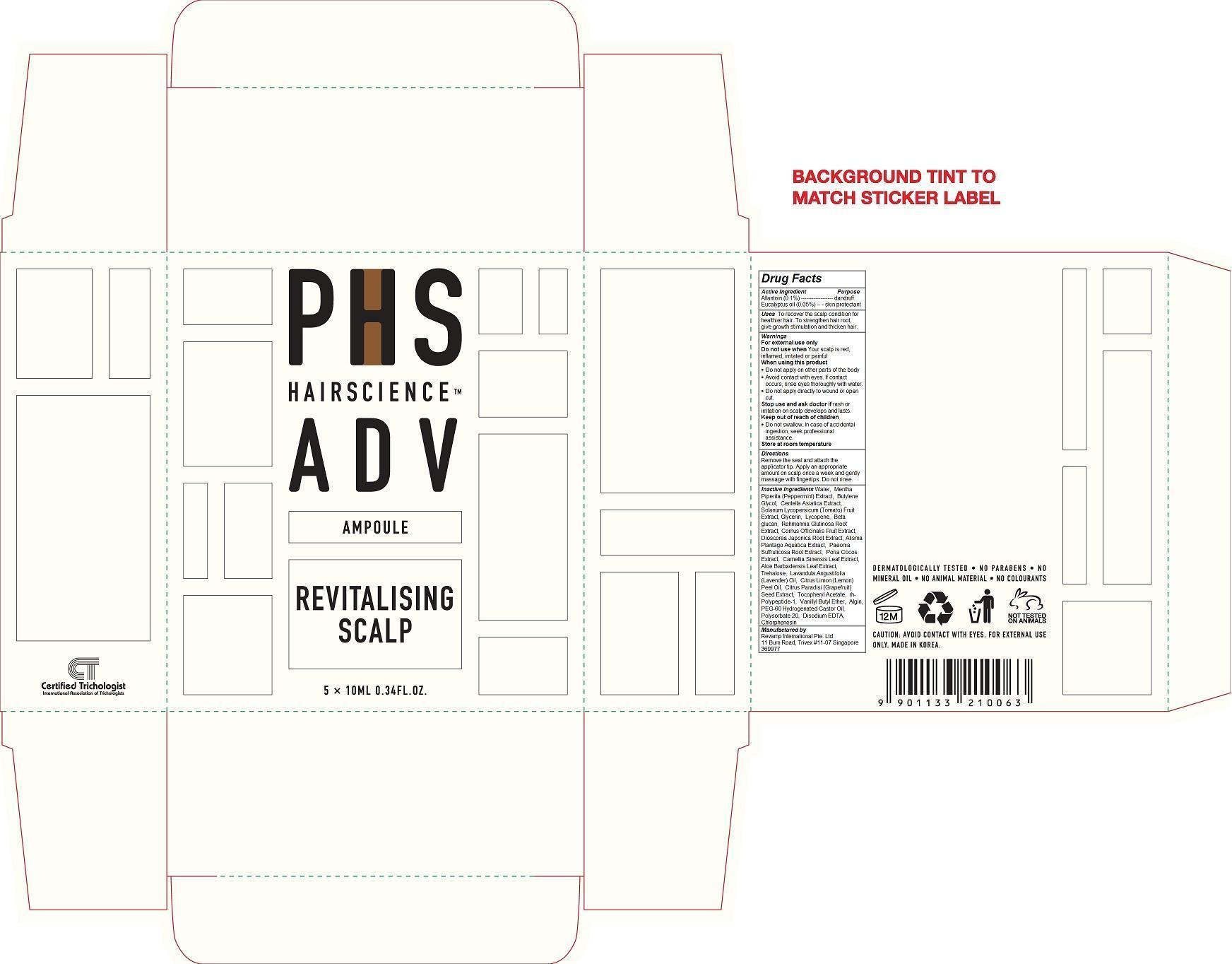
Label: PHS HAIR SCIENCE ADV REVITALIZING SCALP AMPOULE- eucalyptus oil, allantoin liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 69149-103-01, 69149-103-02 - Packager: Revamp International Pte. Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated September 17, 2014
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
PHS Hair Science Adv Revitalizing Scalp Ampoule
Keep out of reach of children
Do not swallow. In case of accidental ingestion, seek professional assistance.To recover the scalp condition for healthier hair. To strengthen hair root, give growth stimulation and thicken hair.
For external use only
Do not use when Your scalp is red, inflamed, irritated or painful When using this product
Do not apply on other parts of the body
Avoid contact with eyes. If contact occurs, rinse eyes thoroughly with water.
Do not apply directly to wound or open cut.
Stop use and ask doctor if rash or irritation on scalp develops and lasts.
Store at room temperatureRemove the seal and attach the applicator tip. Apply an appropriate amount on scalp once a week and gently massage with fingertips. Do not rinse.
Water, Mentha Piperita (Peppermint) Extract, Butylene Glycol, Centella Asiatica Extract, Solanum Lycopersicum (Tomato) Fruit Extract, Glycerin, Lycopene, Beta glucan, Rehmannia Glutinosa Root Extract, Cornus Officinalis Fruit Extract, Dioscorea Japonica Root Extract, Alisma Plantago Aquatica Extract, Paeonia Suffruticosa Root Extract, Poria Cocos Extract, Camellia Sinensis Leaf Extract, Aloe Barbadensis Leaf Extract, Trehalose, Lavandula Angustifolia (Lavender) Oil, Citrus Limon (Lemon) Peel Oil, Citrus Paradisi (Grapefruit) Seed Extract, Tocopheryl Acetate, rh-Polypeptide-1, Vanillyl Butyl Ether, Algin, PEG-60 Hydrogenated Castor Oil, Polysorbate 20, Disodium EDTA, Chlorphenesin
-
INGREDIENTS AND APPEARANCE
PHS HAIR SCIENCE ADV REVITALIZING SCALP AMPOULE
eucalyptus oil, allantoin liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69149-103 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength EUCALYPTUS OIL (UNII: 2R04ONI662) (EUCALYPTUS OIL - UNII:2R04ONI662) EUCALYPTUS OIL 0.05 in 10 mL ALLANTOIN (UNII: 344S277G0Z) (ALLANTOIN - UNII:344S277G0Z) ALLANTOIN 0.1 in 10 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) MENTHA PIPERITA (UNII: 79M2M2UDA9) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) CENTELLA ASIATICA (UNII: 7M867G6T1U) TOMATO (UNII: Z4KHF2C175) GLYCERIN (UNII: PDC6A3C0OX) LYCOPENE (UNII: SB0N2N0WV6) REHMANNIA GLUTINOSA ROOT (UNII: 1BEM3U6LQQ) CORNUS OFFICINALIS FRUIT (UNII: 23NL8NQ187) DIOSCOREA JAPONICA ROOT (UNII: I43FCF3356) ALISMA PLANTAGO-AQUATICA WHOLE (UNII: OX88Q81K8S) PAEONIA SUFFRUTICOSA ROOT (UNII: 7M7E9A2C8J) FU LING (UNII: XH37TWY5O4) GREEN TEA LEAF (UNII: W2ZU1RY8B0) ALOE VERA LEAF (UNII: ZY81Z83H0X) TREHALOSE (UNII: B8WCK70T7I) LAVENDER OIL (UNII: ZBP1YXW0H8) LEMON OIL (UNII: I9GRO824LL) CITRUS PARADISI SEED (UNII: 12F08874Y7) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) BASIC FIBROBLAST GROWTH FACTOR (HUMAN) (UNII: S3529G9M9V) VANILLYL BUTYL ETHER (UNII: S2ULN37C9R) SODIUM ALGINATE (UNII: C269C4G2ZQ) PEG-60 HYDROGENATED CASTOR OIL (UNII: 02NG325BQG) POLYSORBATE 20 (UNII: 7T1F30V5YH) EDETATE DISODIUM (UNII: 7FLD91C86K) CHLORPHENESIN (UNII: I670DAL4SZ) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69149-103-02 5 in 1 CARTON 1 NDC:69149-103-01 10 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 09/17/2014 Labeler - Revamp International Pte. Ltd. (595376066) Registrant - Revamp International Pte. Ltd. (595376066) Establishment Name Address ID/FEI Business Operations Revamp International Pte. Ltd. 595376066 manufacture(69149-103)