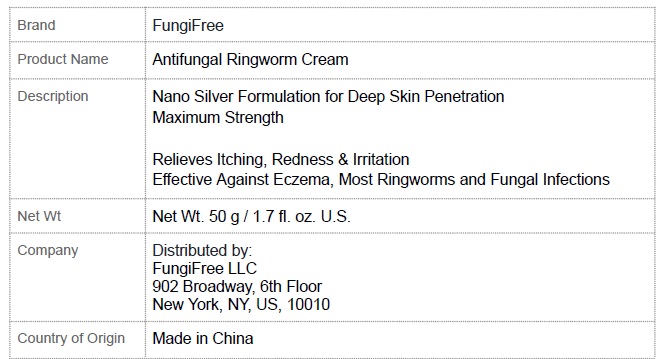
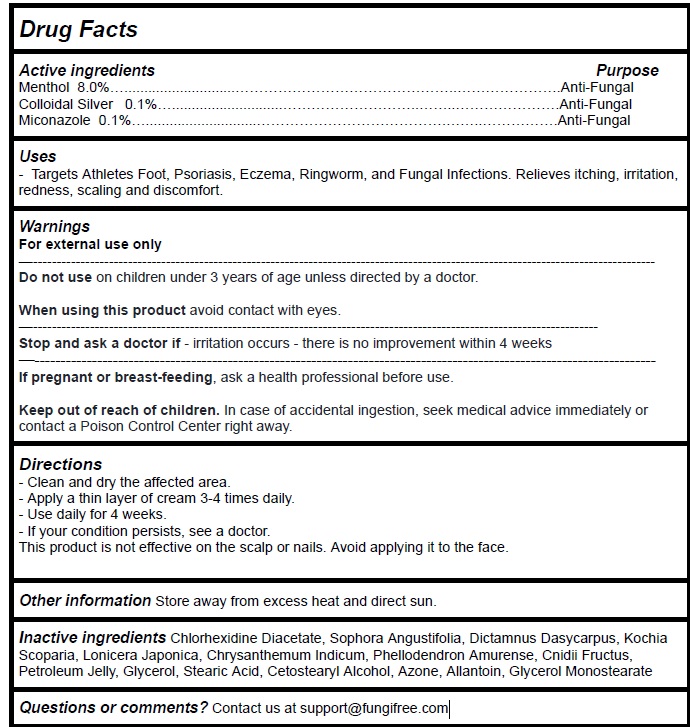
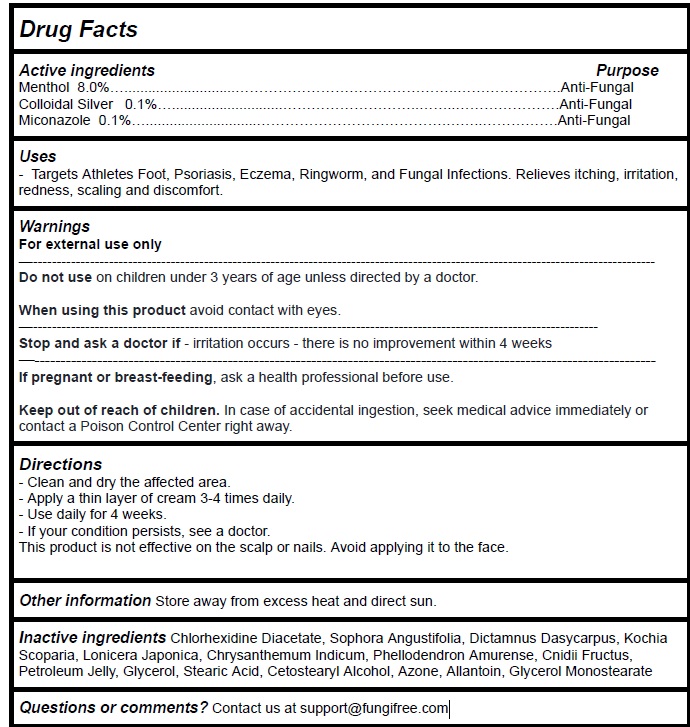
Label: FUNGIFREE ANTIFUNGAL RINGWORM CREAM- menthol,colloidal silver,miconazole cream
- NDC Code(s): 83364-003-01
- Packager: YITONGBADA (SHENZHEN) INTERNATIONAL TRADE CO., LTD
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated March 15, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Active Ingredient Purpose
Menthol 8.0%…............................…………………..………………….Anti-Fungal - Colloidal Silver 0.1%…............................……..…..………………….Anti-Fungal - Miconazole ...
- PURPOSE
-
Uses
Targets Athletes Foot, Psoriasis, Eczema, Ringworm, and Fungal Infections. Relieves itching, irritation, redness, scaling and discomfort.
-
Warnings
For external use only - Do not use onchildren under 3 years of age unless directed by a doctor. When using this productavoid contact with eyes. Stop and ask a doctor if- irritation ...
- KEEP OUT OF REACH OF CHILDREN
-
Directions
Clean and dry the affected area. Apply a thin layer of cream 3-4 times daily. Use daily for 4 weeks. If your condition persists, see a doctor. This product is not effective on the scalp or nails ...
-
Other information
Store away from excess heat and direct sun.
-
Inactive ingredients
Chlorhexidine Diacetate, Sophora Angustifolia, Dictamnus Dasycarpus, Kochia Scoparia, Lonicera Japonica, Chrysanthemum Indicum, Phellodendron Amurense, Cnidii Fructus, Petroleum Jelly, Glycerol ...
-
Questions or comments?
Contact us at support@fungifree.com
-
Product label
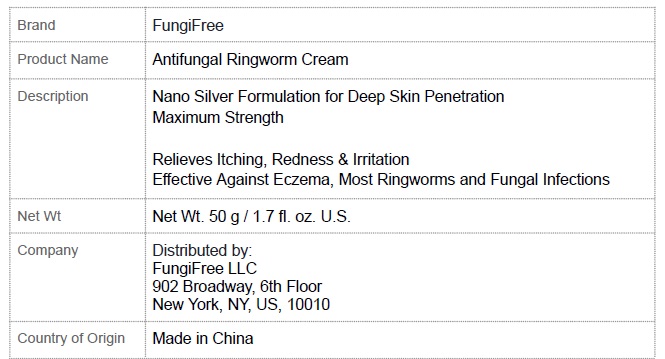 ...
... -
INGREDIENTS AND APPEARANCEProduct Information