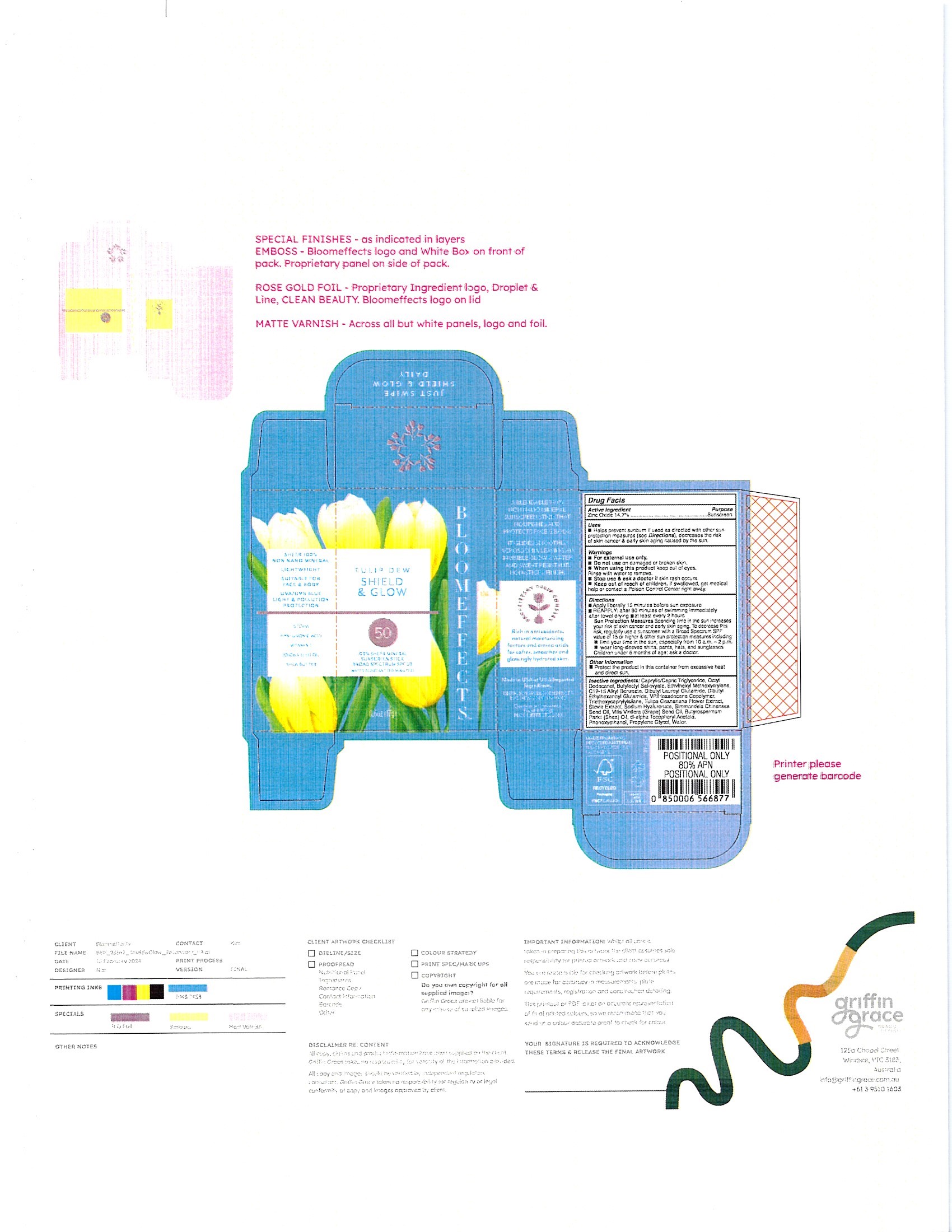
Label: TULIP DEW SHIELD GLOW- zinc oxide stick
- NDC Code(s): 82548-2420-1
- Packager: Bloom effects, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated May 30, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- WHEN USING
- WARNINGS
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- DO NOT USE
-
INACTIVE INGREDIENT
Inactive Ingredients
Caprylic/Capric Triglyceride
Butyloctyl Salicylate
Butyrospermum Parkii(Shea) Oil
C12-15 Alkyl Benzoate
Dibutyl Ethylhexanoyl Glutamide
Dibutyl Lauroyl Glutamide
dl-alpha Tocopheryl Acetate
Ethylhexyl Methoxycrylene
Octyl Dodecanol
Phenoxyethanol
Propylene glycol
Simmondsia Chinenses Seed oil
Stevia Extract
Sodium hyaluronate
Triethoxycaprylylsilane
Tulipa Gesneriana Flower Extract
Vitis Vinifera (Grape) Seed Oil
VP/Hexadecene Copolymer
Water - PURPOSE
- DOSAGE & ADMINISTRATION
- INDICATIONS & USAGE
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
TULIP DEW SHIELD GLOW
zinc oxide stickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82548-2420 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 14.7 g in 100 g Inactive Ingredients Ingredient Name Strength .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) SHEANUT OIL (UNII: O88E196QRF) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) DIBUTYL LAUROYL GLUTAMIDE (UNII: 3V7K3IA58X) DIBUTYL ETHYLHEXANOYL GLUTAMIDE (UNII: 0IAF2L30VS) PHENOXYETHANOL (UNII: HIE492ZZ3T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) STEVIA REBAUDIUNA LEAF (UNII: 6TC6NN0876) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) ETHYLHEXYL METHOXYCRYLENE (UNII: S3KFG6Q5X8) JOJOBA OIL (UNII: 724GKU717M) VINYLPYRROLIDONE/HEXADECENE COPOLYMER (UNII: KFR5QEN0N9) WATER (UNII: 059QF0KO0R) 2-HEXYLDODECANOL (UNII: NR6259U734) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) HYALURONATE SODIUM (UNII: YSE9PPT4TH) GRAPE SEED OIL (UNII: 930MLC8XGG) MAGNOLIA LILIIFLORA FLOWER BUD (UNII: K99MFN37XX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82548-2420-1 17 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 04/02/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/01/2024 Labeler - Bloom effects, Inc. (096991853) Establishment Name Address ID/FEI Business Operations Inspec Solutions 081030372 manufacture(82548-2420)