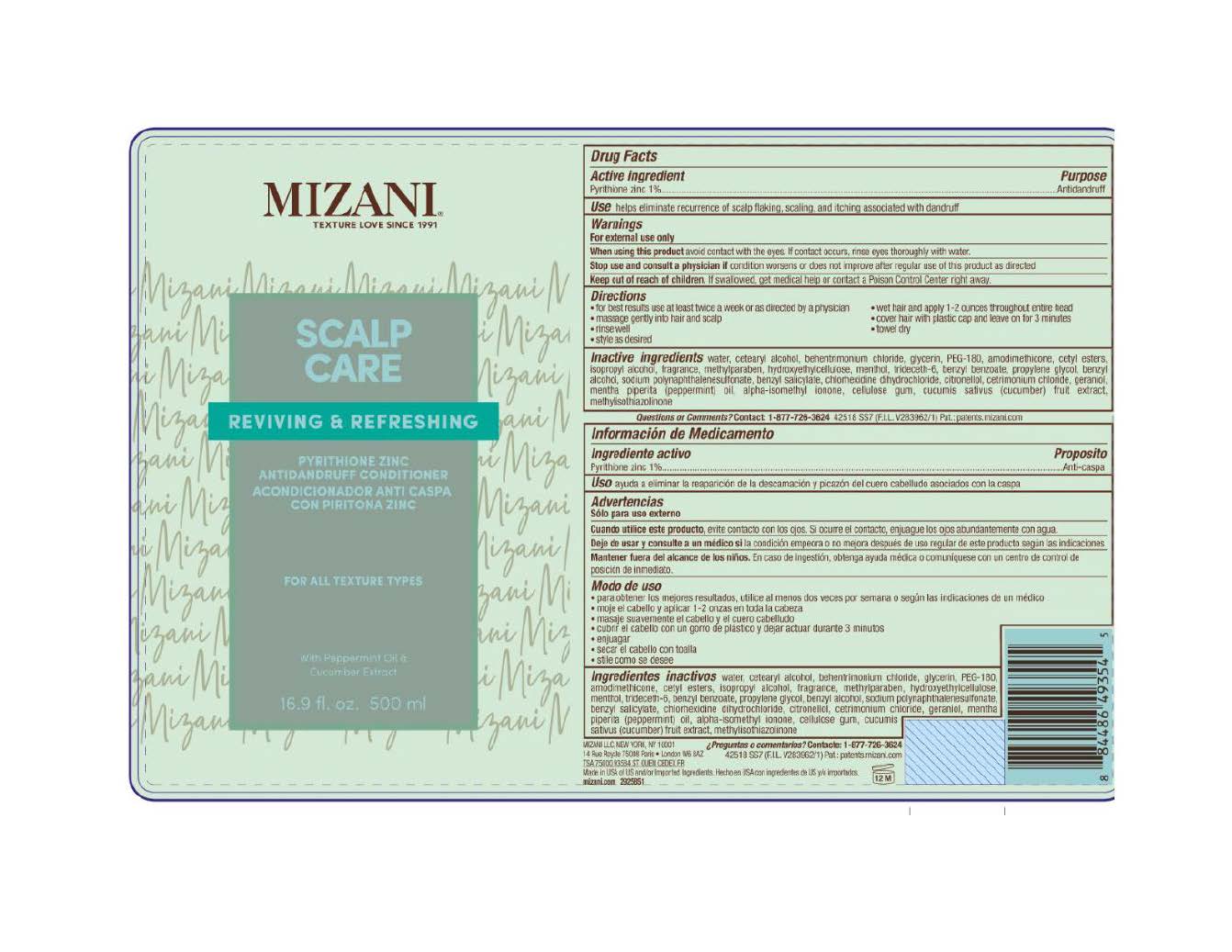
Label: MIZANI SCALP CARE CONDITIONER ANTIDANDRUFF CONDITIONER- pyrithione zinc lotion
- NDC Code(s): 49967-389-01, 49967-389-02
- Packager: L'Oreal USA Products Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 11, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Use
- Warnings
- When using this product
- Stop use and consult a physician if
- Keep out of reach of children.
- Directions
-
Inactive ingredients
water, cetearyl alcohol, behentrimonium chloride, glycerin, PEG-180, amodimethicone, cetyl esters, isopropyl alcohol, fragrance, methylparaben, hydroxyethylcellulose, menthol, trideceth-6, benzyl benzoate, propylene glycol, benzyl alcohol, sodium polynaphthalenesulfonate, benzyl salicylate, chlorhexidine dihydrochloride, citronellol, cetrimonium chloride, geraniol, mentha piperita (peppermint) oil, alpha-isomethyl ionone, cellulose gum, cucumis sativus (cucumber) fruit extract, methylisothiazolinone
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MIZANI SCALP CARE CONDITIONER ANTIDANDRUFF CONDITIONER
pyrithione zinc lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49967-389 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PYRITHIONE ZINC (UNII: R953O2RHZ5) (PYRITHIONE ZINC - UNII:R953O2RHZ5) PYRITHIONE ZINC 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) BEHENTRIMONIUM CHLORIDE (UNII: X7GNG3S47T) POLYETHYLENE GLYCOL 8000 (UNII: Q662QK8M3B) AMODIMETHICONE (1300 CST) (UNII: 3V7U636DWN) CETYL ESTERS WAX (UNII: D072FFP9GU) ISOPROPYL ALCOHOL (UNII: ND2M416302) METHYLPARABEN (UNII: A2I8C7HI9T) HYDROXYETHYL CELLULOSE, UNSPECIFIED (UNII: T4V6TWG28D) MENTHOL (UNII: L7T10EIP3A) TRIDECETH-6 (UNII: 3T5PCR2H0C) BENZYL BENZOATE (UNII: N863NB338G) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) BENZYL ALCOHOL (UNII: LKG8494WBH) BENZYL SALICYLATE (UNII: WAO5MNK9TU) CHLORHEXIDINE HYDROCHLORIDE (UNII: E64XL9U38K) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) CETRIMONIUM CHLORIDE (UNII: UC9PE95IBP) GERANIOL (UNII: L837108USY) PEPPERMINT OIL (UNII: AV092KU4JH) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED (UNII: K679OBS311) CUCUMBER (UNII: YY7C30VXJT) METHYLISOTHIAZOLINONE (UNII: 229D0E1QFA) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49967-389-01 500 mL in 1 BOTTLE; Type 0: Not a Combination Product 06/01/2008 2 NDC:49967-389-02 1000 mL in 1 BOTTLE; Type 0: Not a Combination Product 06/01/2008 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M032 06/01/2008 Labeler - L'Oreal USA Products Inc (002136794) Establishment Name Address ID/FEI Business Operations L'Oreal USA, Inc. 960317444 manufacture(49967-389)