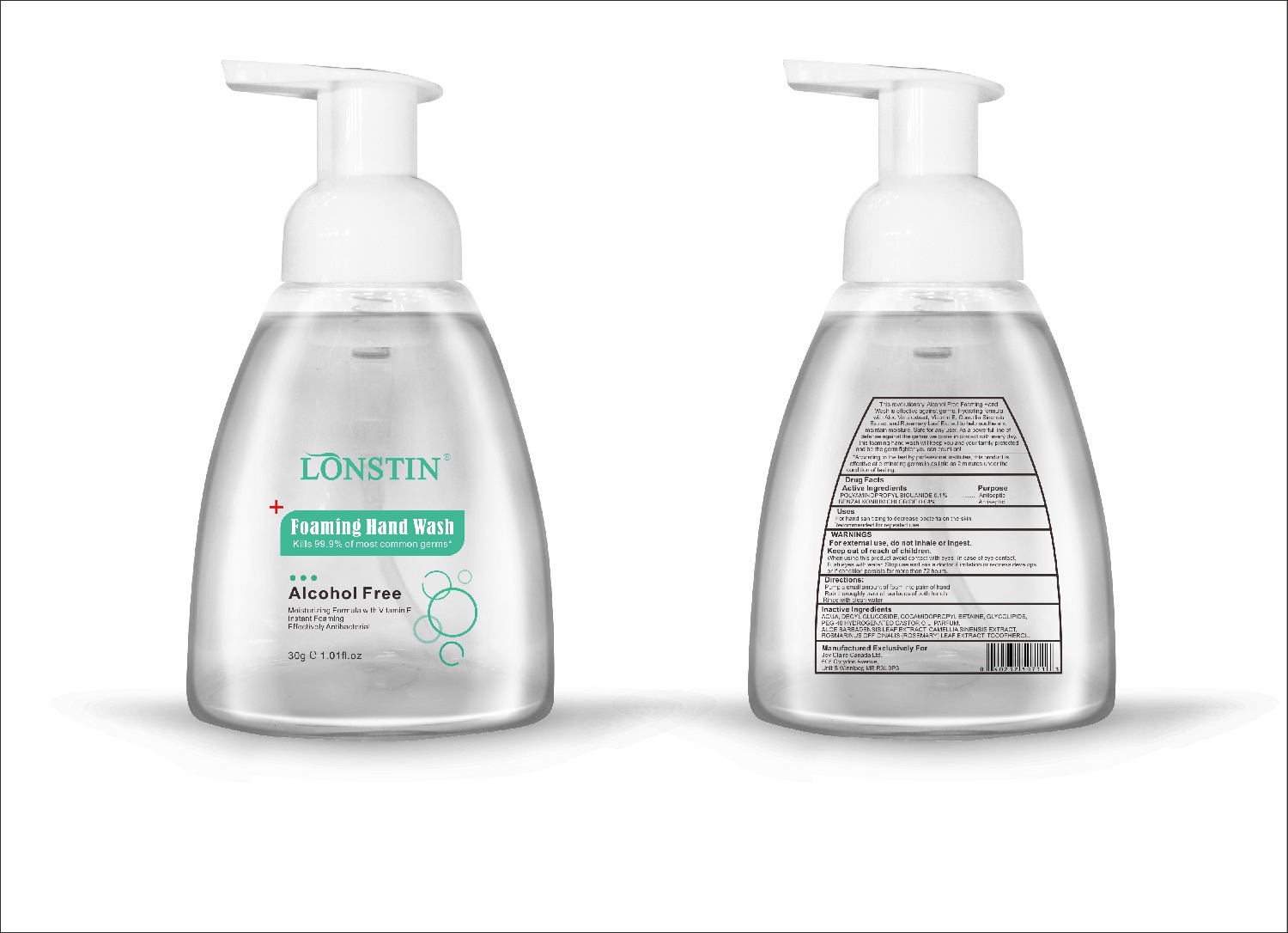
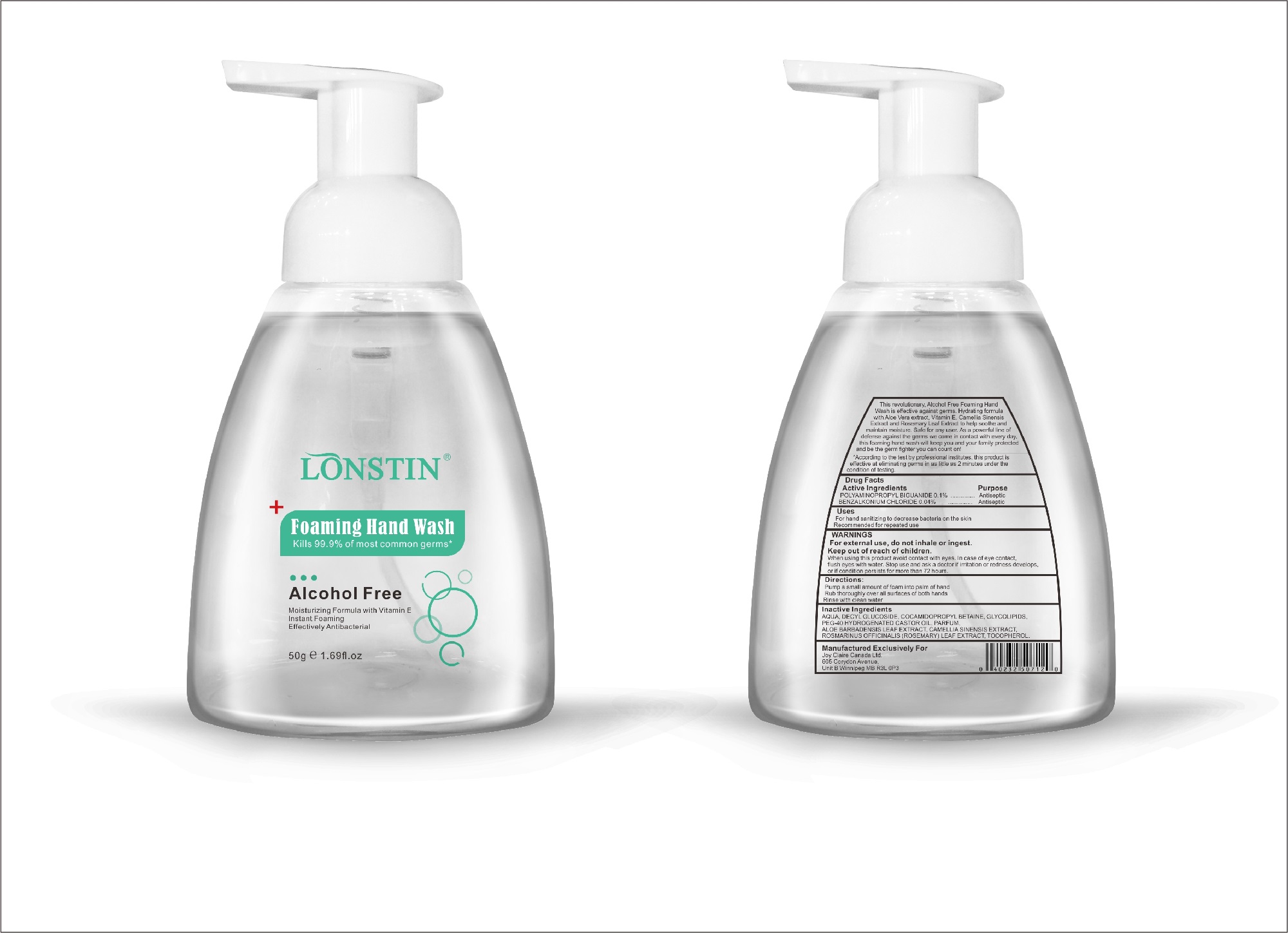
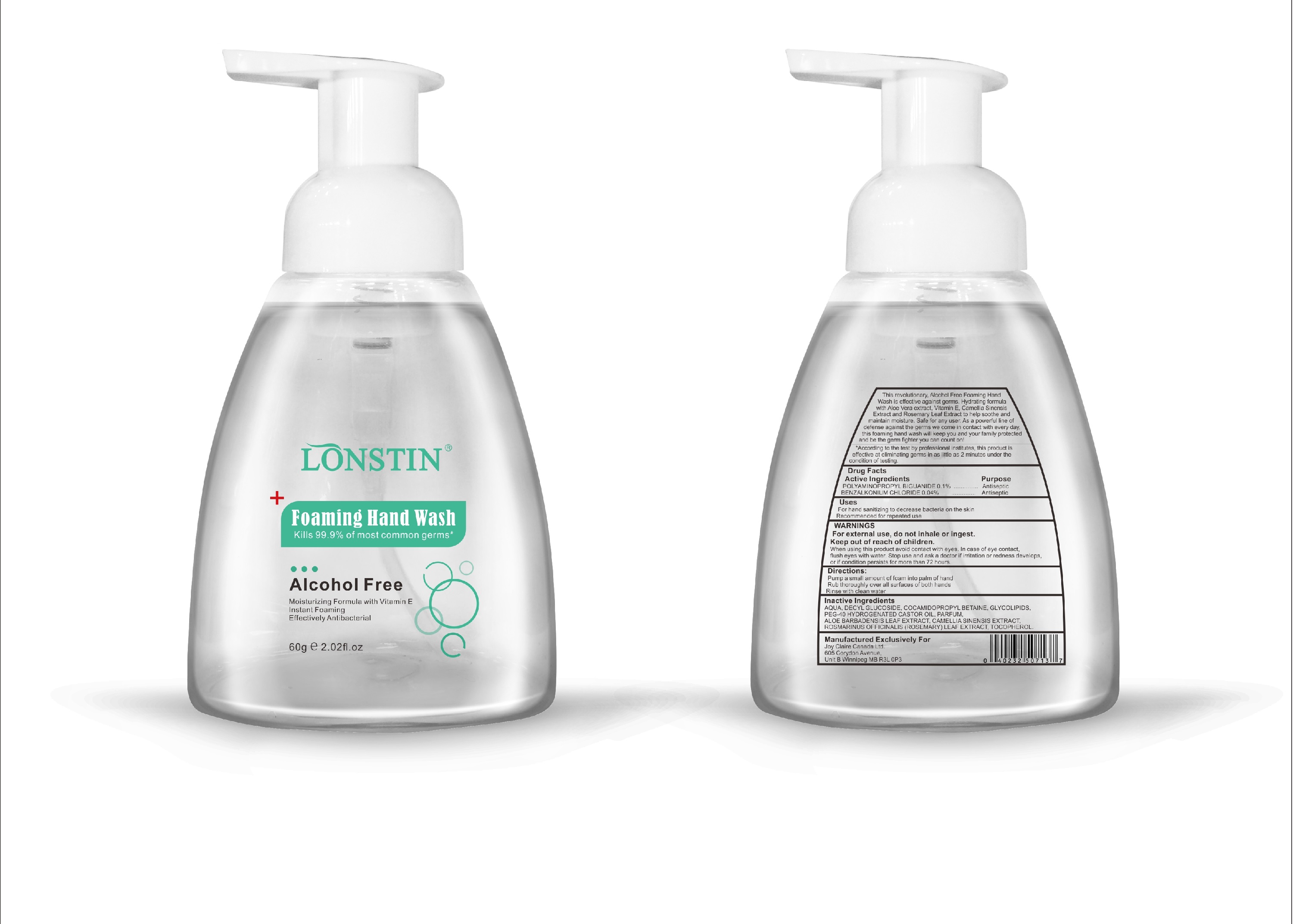
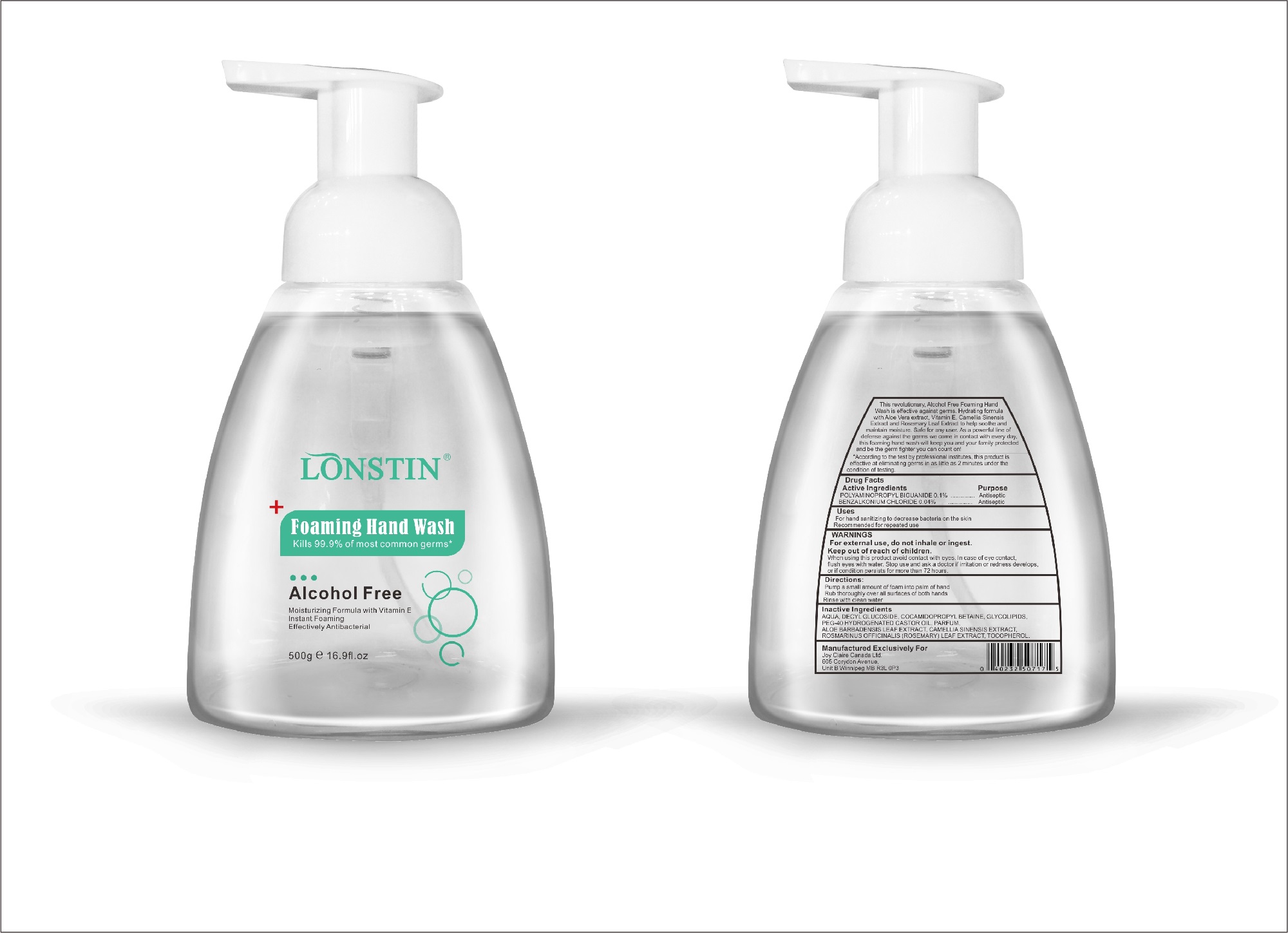
Label: FOAMING HAND WASH liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 74743-007-01, 74743-007-02, 74743-007-03, 74743-007-04, view more74743-007-05, 74743-007-06, 74743-007-07, 74743-007-08 - Packager: Guangzhou Joy Claire Biological Technology Co., Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated April 20, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SUMMARY OF SAFETY AND EFFECTIVENESS
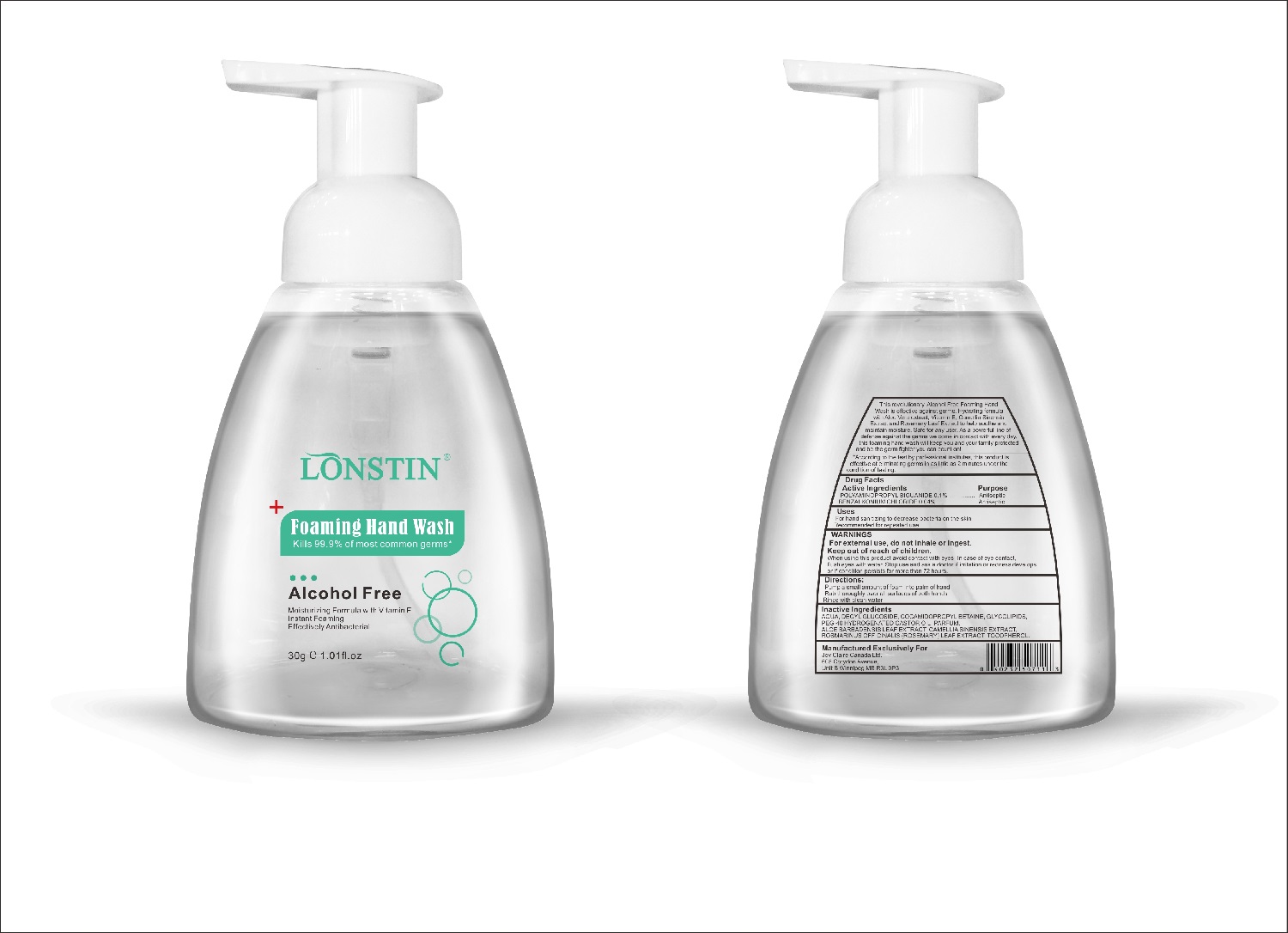
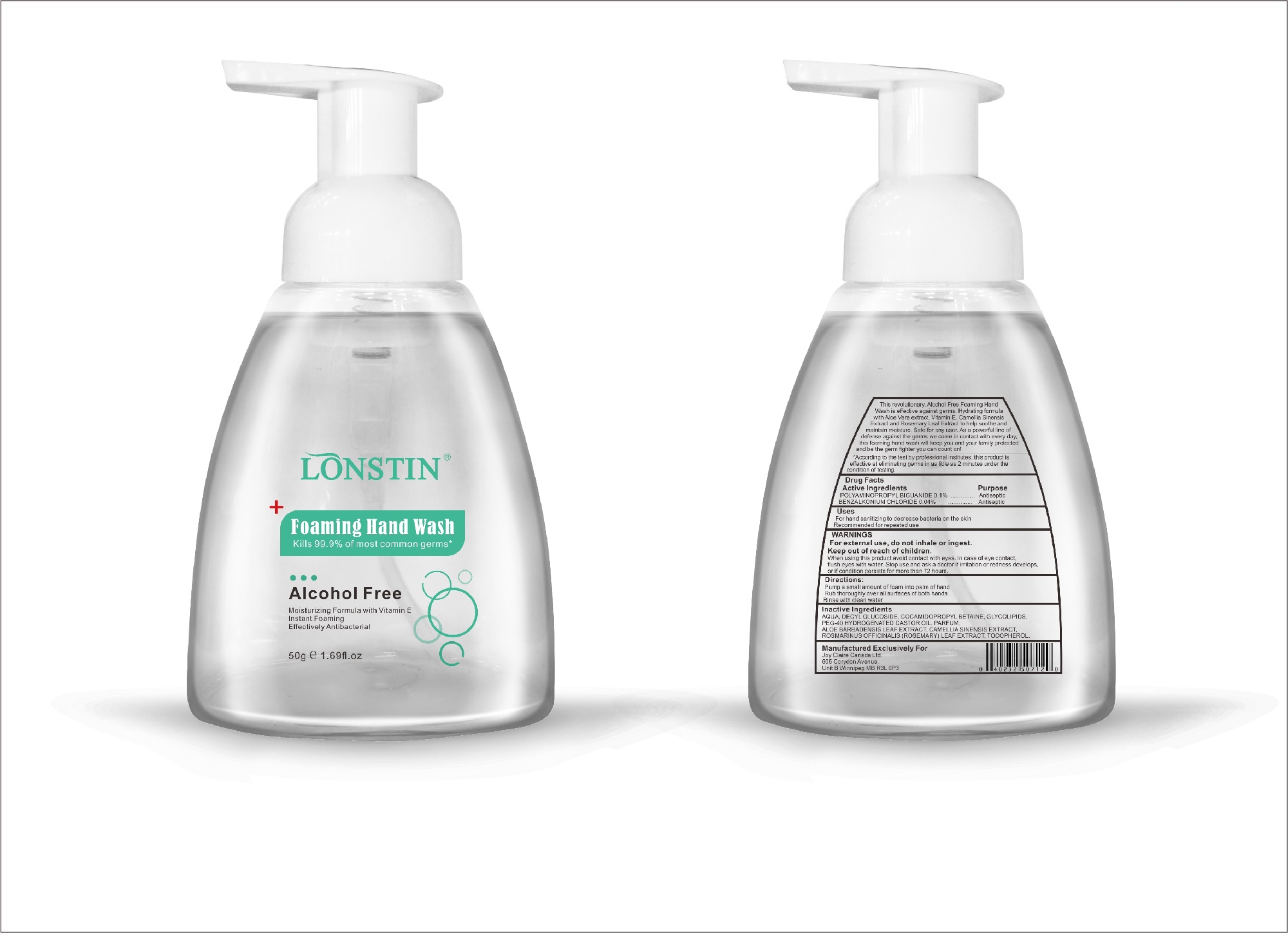
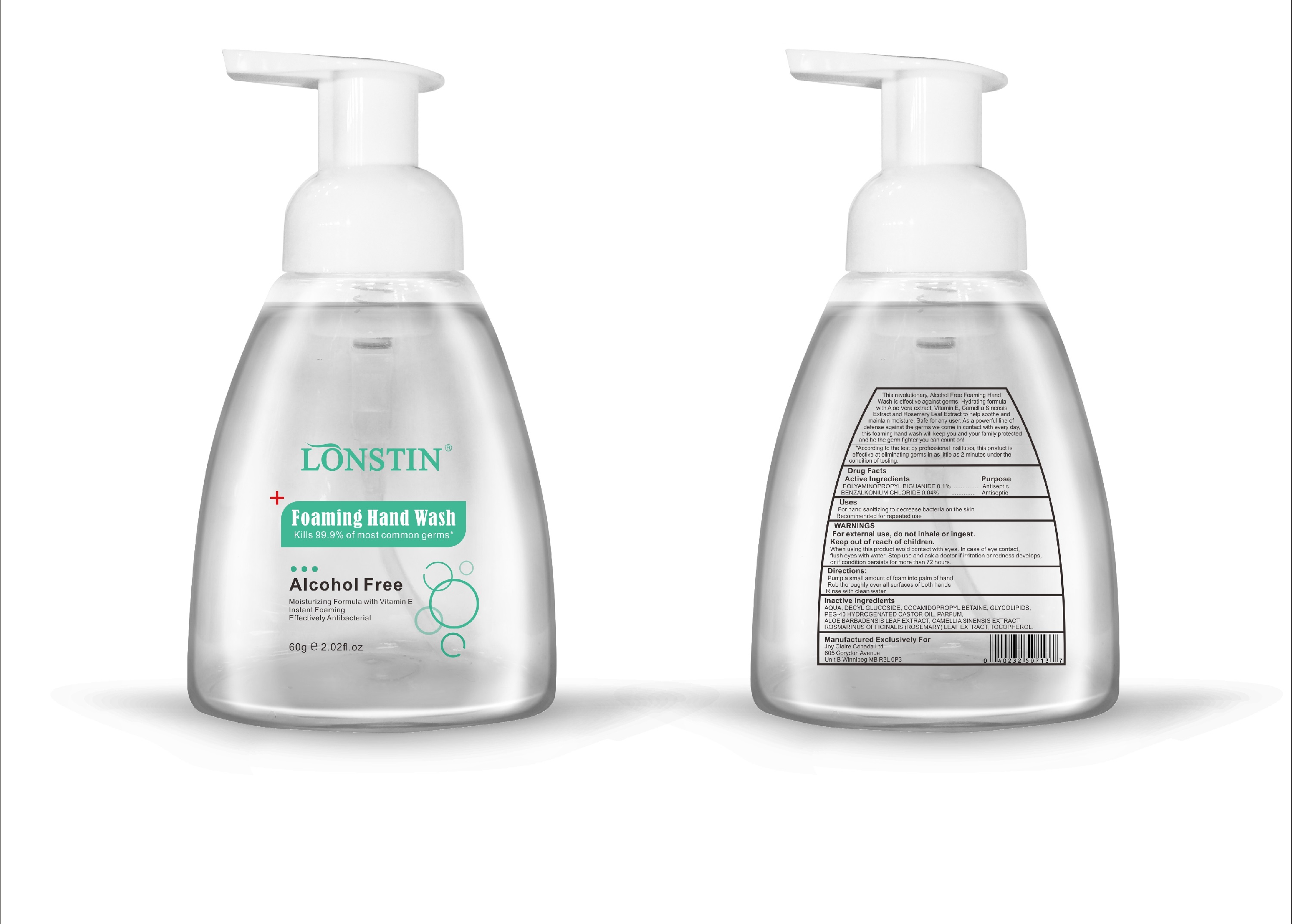
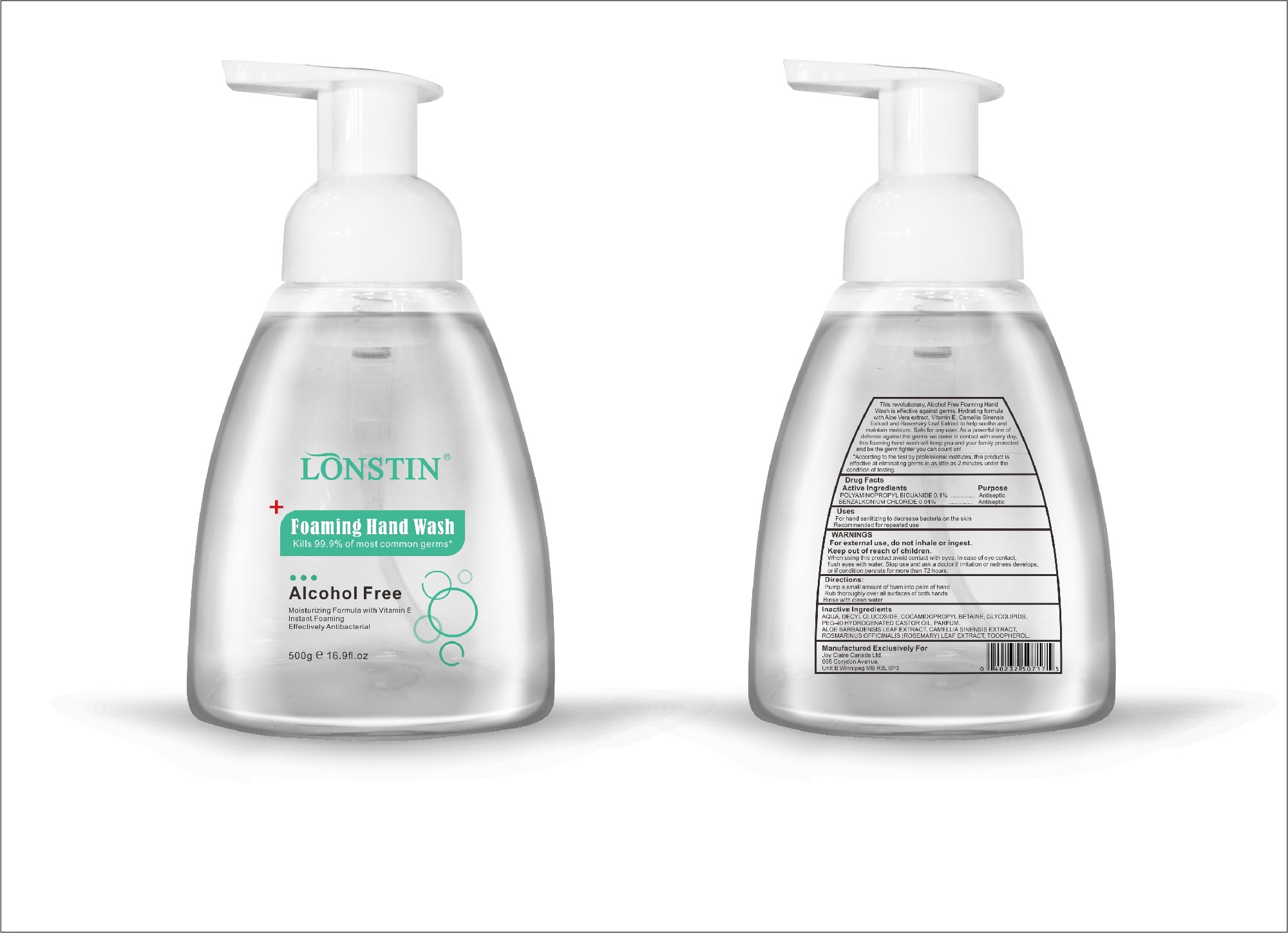
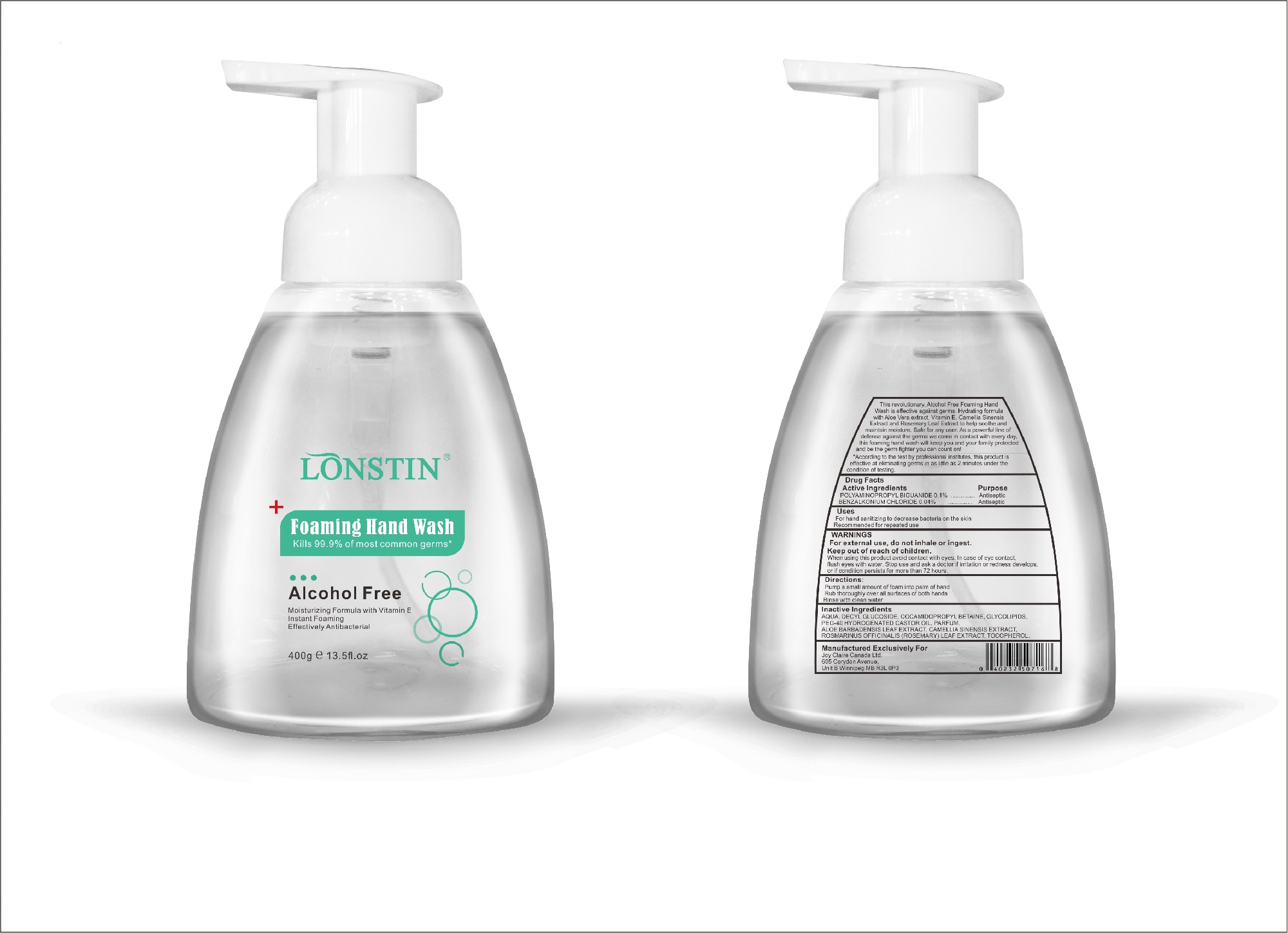
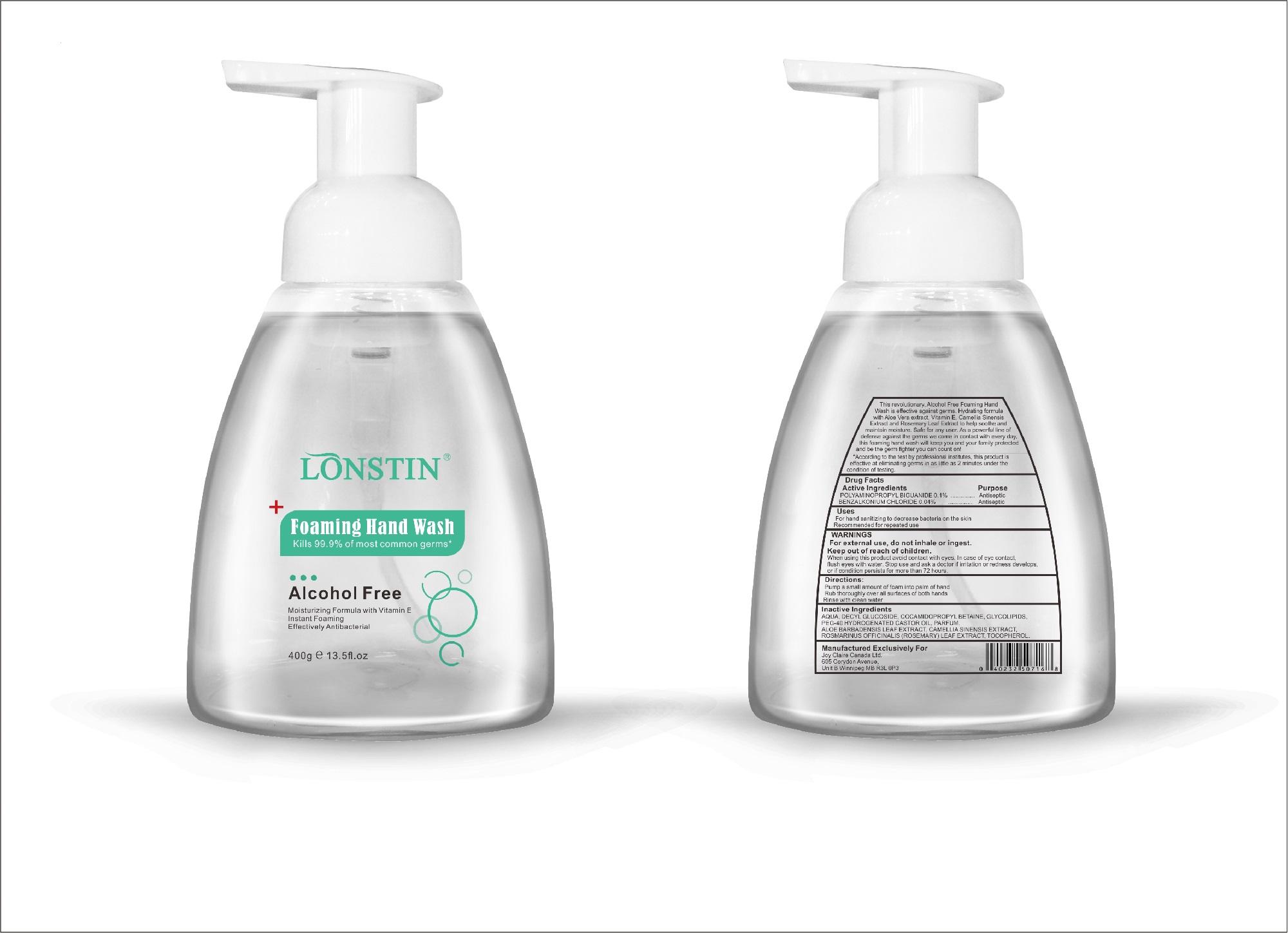
- This revolutionary, Alcohol Free Foaming Hand Wash is effective against germs. Hydrating formula with Aloe Vera extract, Vitamin E. Camellia Sinensis Extract and Rosemary Leaf Extract to help soothe and maintain moisture. Safe for any user. As a powerful line of defense against the germs we come in contact with every day.this foaming hand wash will keep you and your family protected and be the germ fighter you can count on!
- According to the test by professional institutes, this product is effective at eliminating germs in as little as 2 minutes under the condition of testing
- Active Ingredient(s)
- Purpose
- Uses
- Warnings
- KEEP OUT OF REACH OF CHILDREN
- Do not use
-
WHEN USING
When using this product keep out of eyes, ears, and mouth. In case of contact with eyes, rinse eyes thoroughly with water.
Stop use and ask a doctor if irritation or rash occurs. These may be signs of a serious condition.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away. - STOP USE
- Directions
- Other information
- Inactive ingredients
- Package Label - Principal Display Panel
-
INGREDIENTS AND APPEARANCE
FOAMING HAND WASH
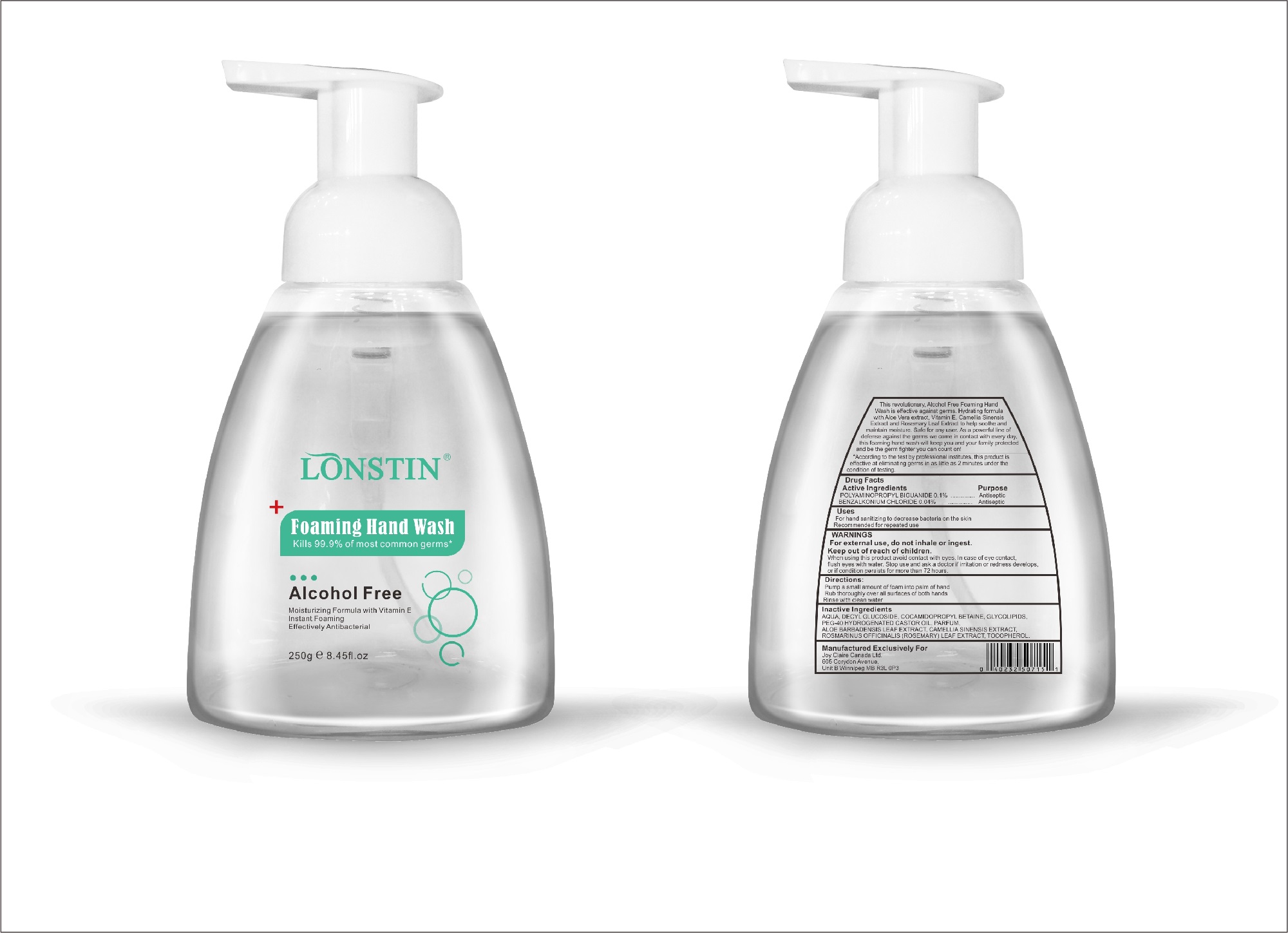
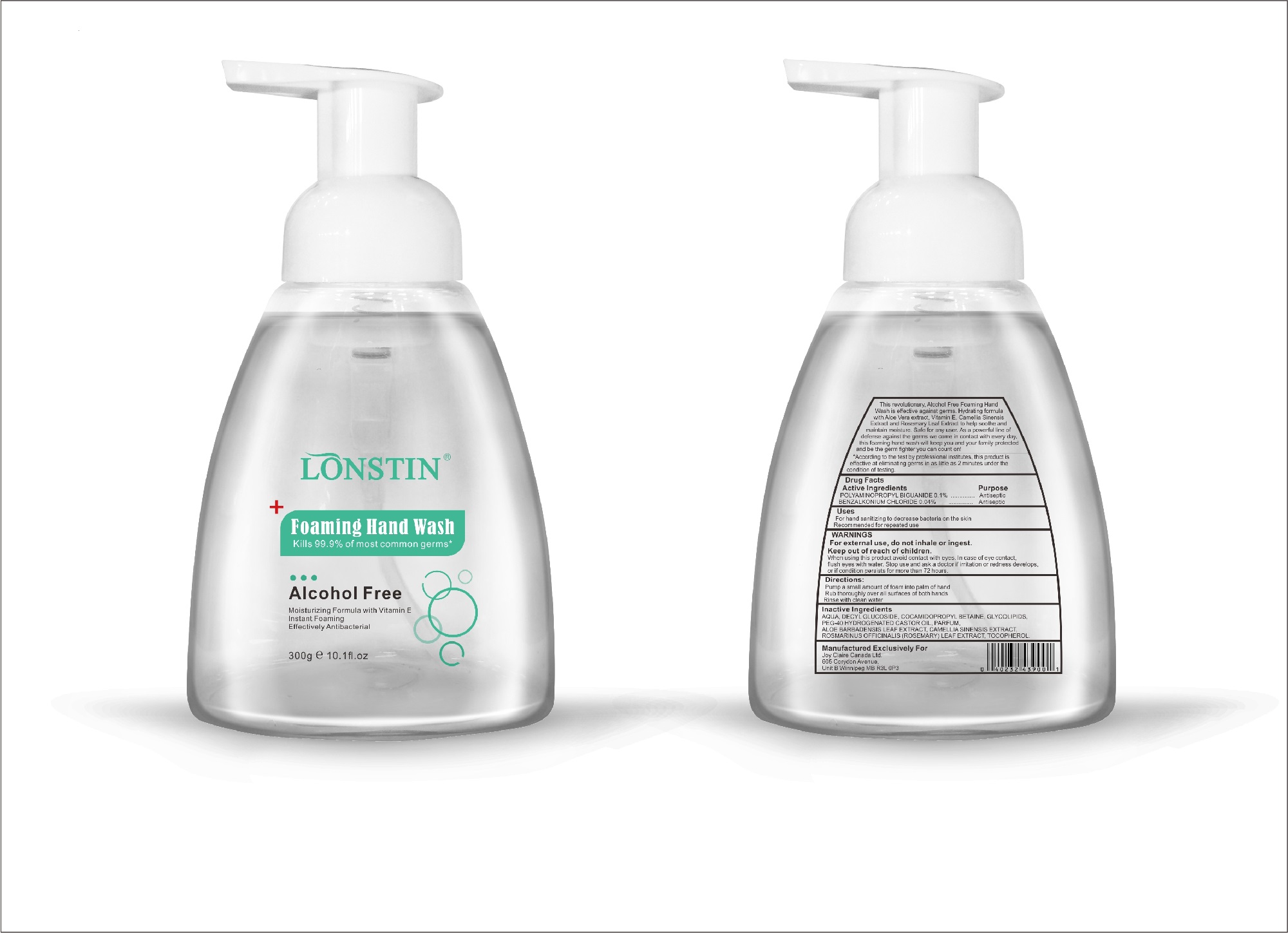
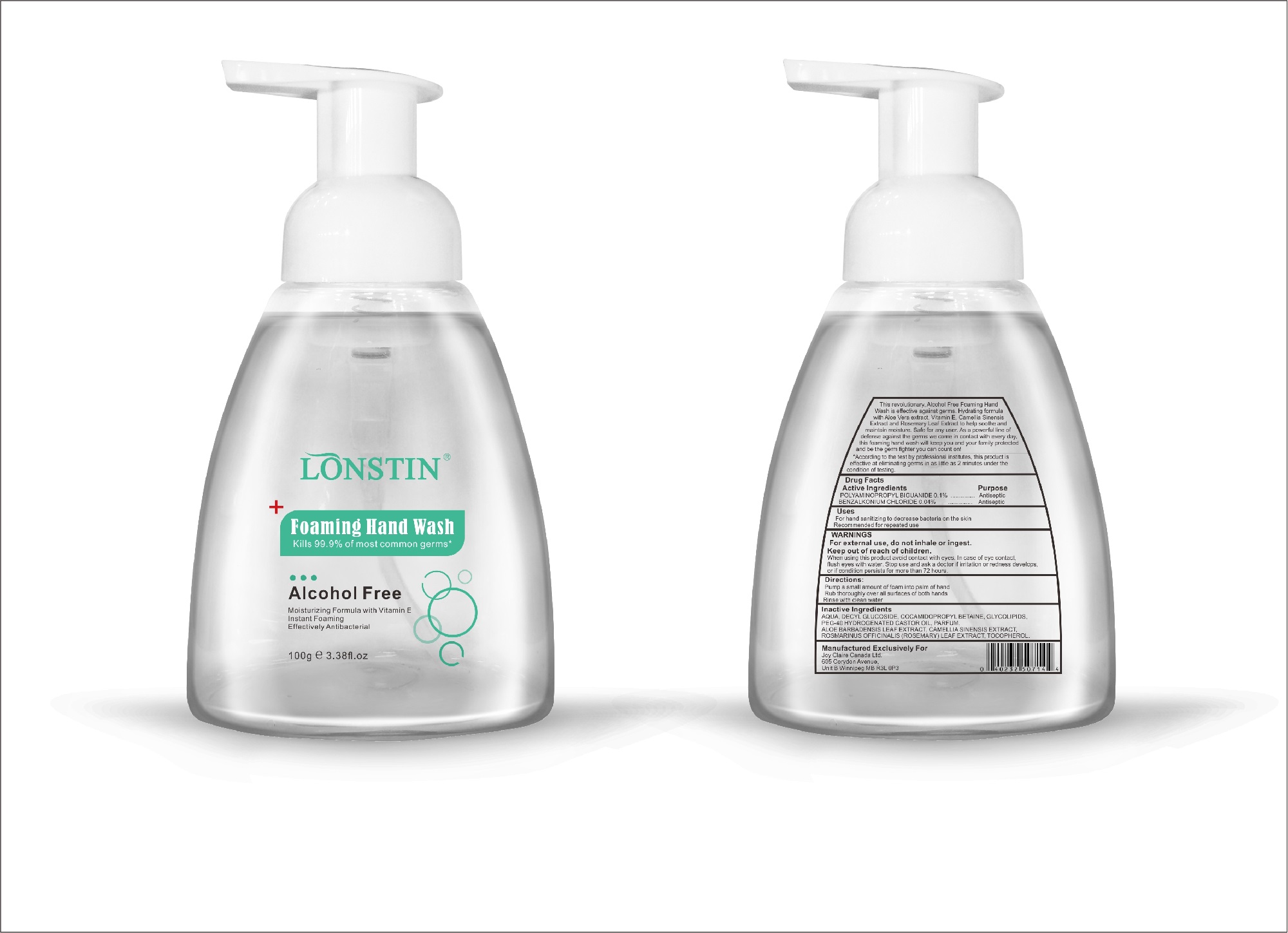
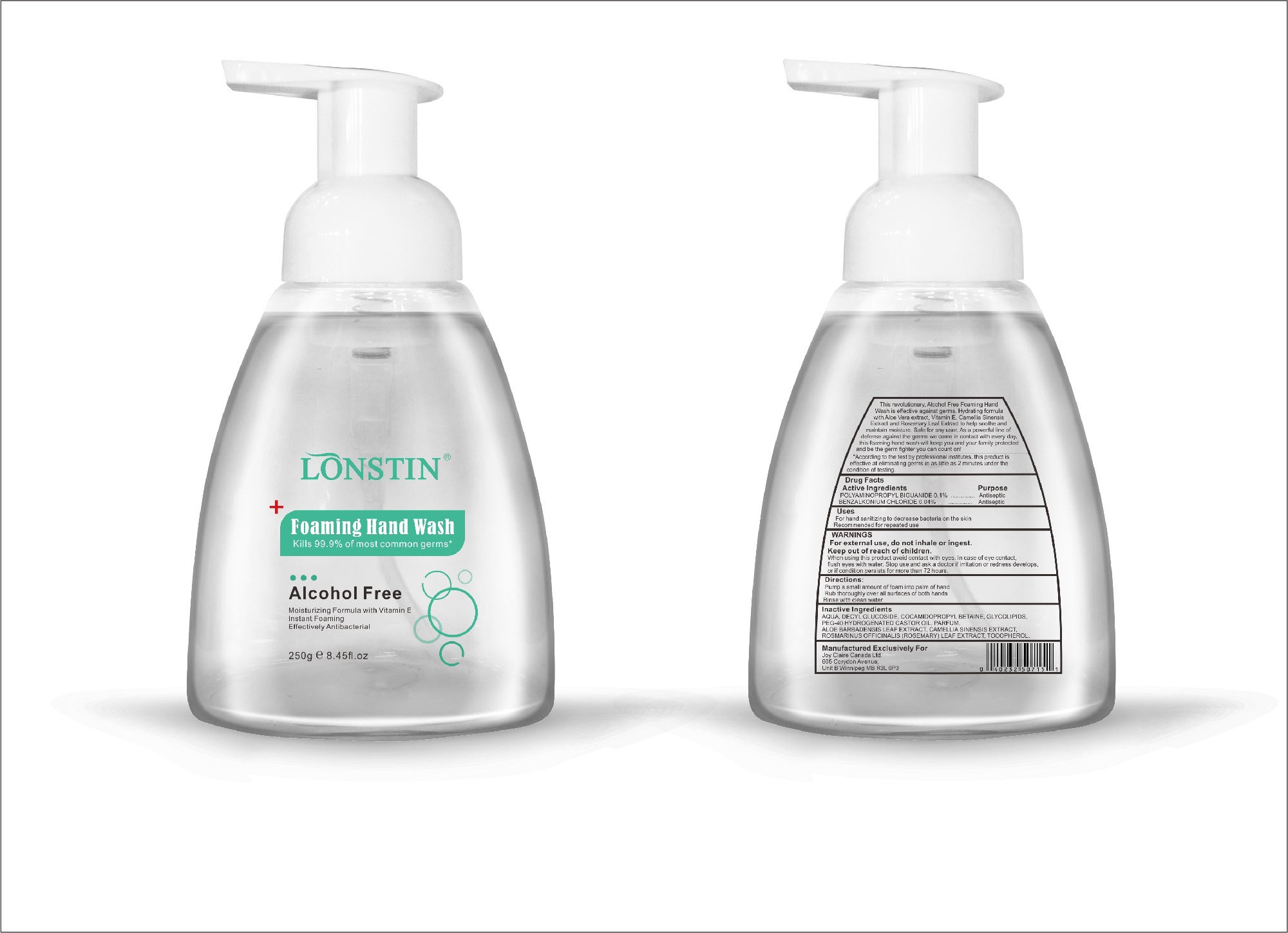
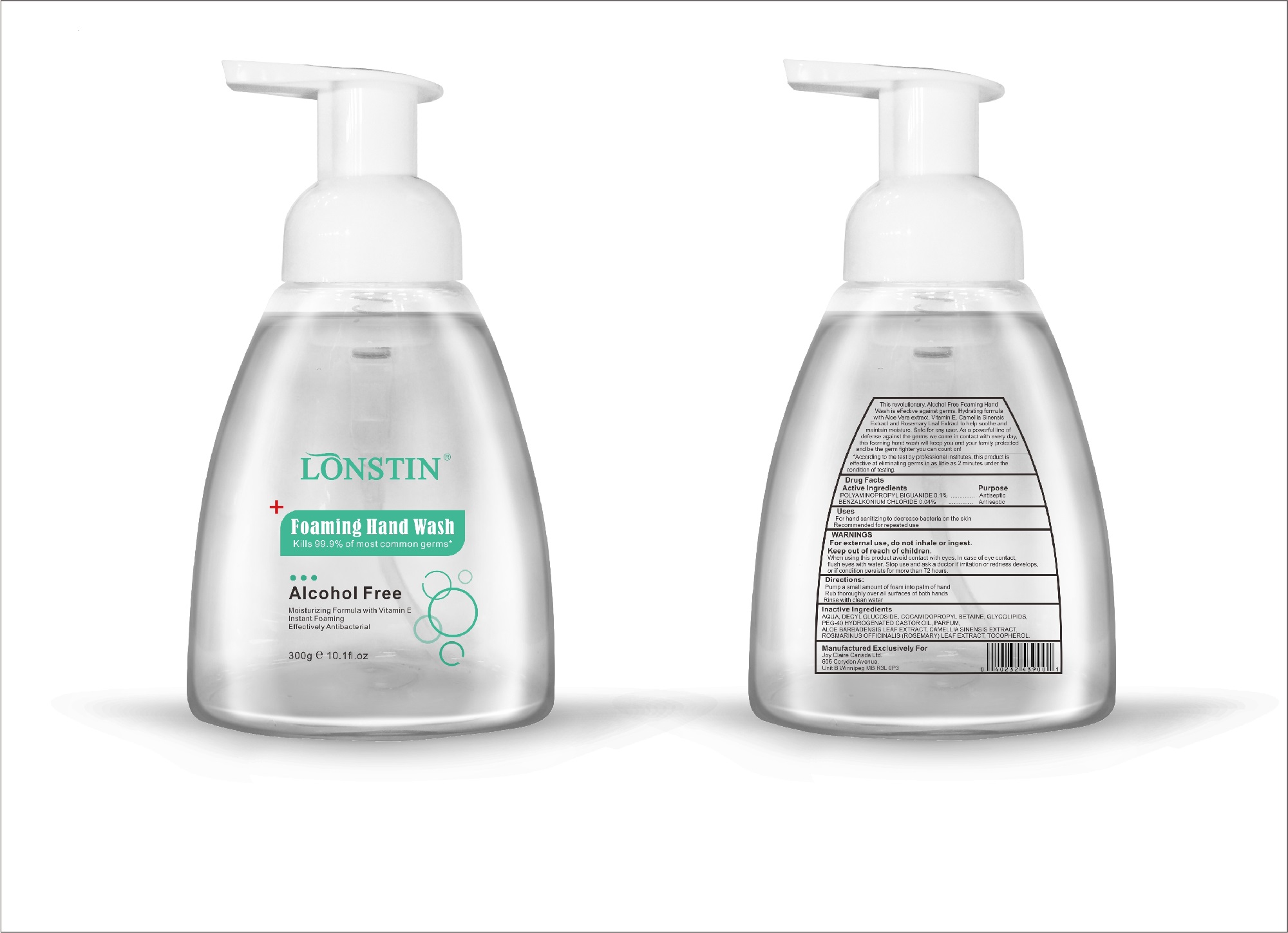
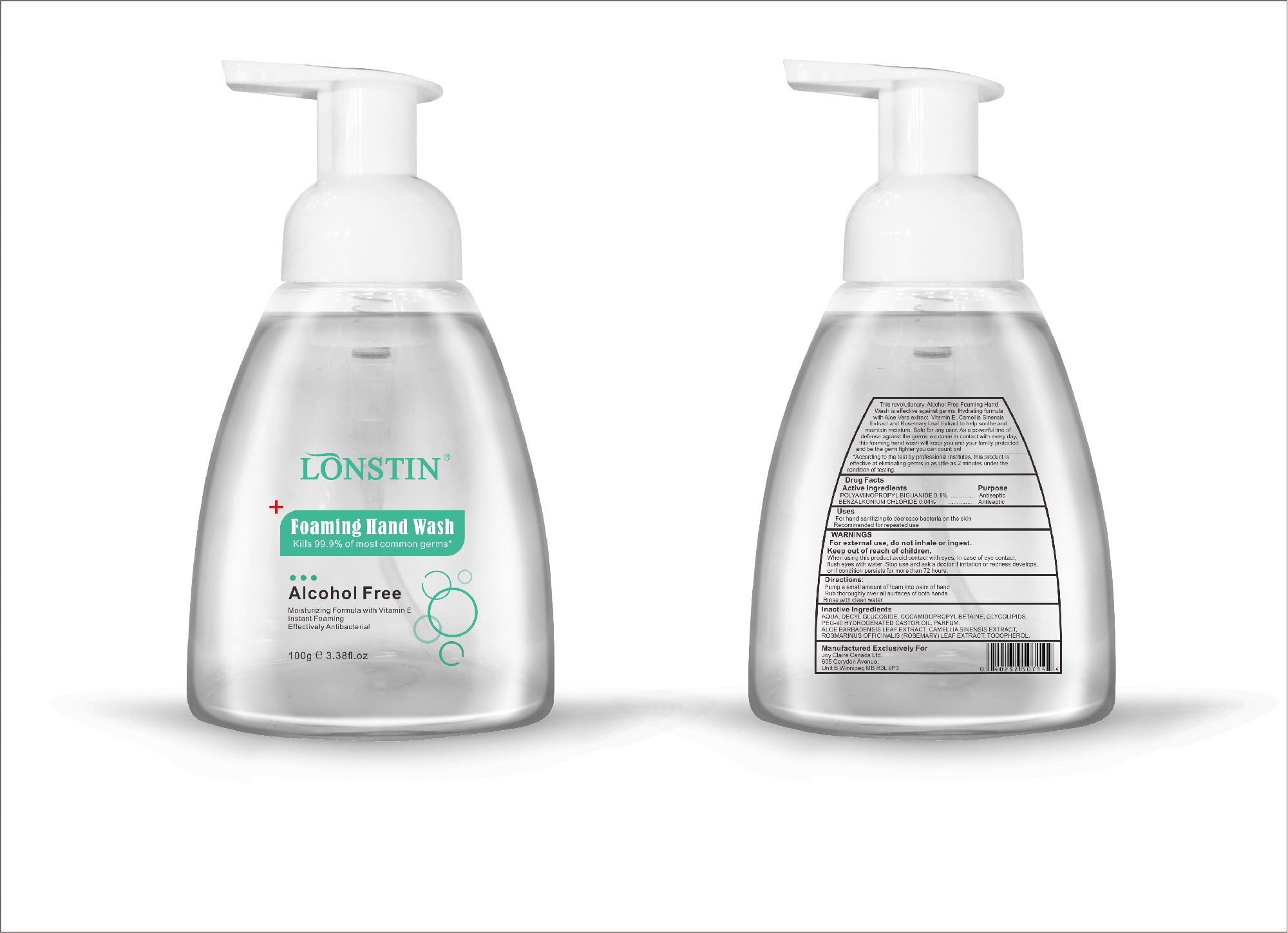
foaming hand wash liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:74743-007 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.04 g in 100 g POLYAMINOPROPYL BIGUANIDE (UNII: DT9D8Z79ET) (POLYAMINOPROPYL BIGUANIDE - UNII:DT9D8Z79ET) POLYAMINOPROPYL BIGUANIDE 0.1 g in 100 g Inactive Ingredients Ingredient Name Strength POLYOXYL 40 HYDROGENATED CASTOR OIL (UNII: 7YC686GQ8F) ALOE (UNII: V5VD430YW9) CAMELLIA SINENSIS SEED (UNII: MRL49Z8AC4) ROSEMARY (UNII: IJ67X351P9) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) DECYL GLUCOSIDE (UNII: Z17H97EA6Y) WATER (UNII: 059QF0KO0R) GLYCOSINE (UNII: D5JUH3HNWF) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:74743-007-01 500 g in 1 BOTTLE; Type 0: Not a Combination Product 04/20/2020 2 NDC:74743-007-02 400 g in 1 BOTTLE; Type 0: Not a Combination Product 04/20/2020 3 NDC:74743-007-03 300 g in 1 BOTTLE; Type 0: Not a Combination Product 04/20/2020 4 NDC:74743-007-04 250 g in 1 BOTTLE; Type 0: Not a Combination Product 04/20/2020 5 NDC:74743-007-05 100 g in 1 BOTTLE; Type 0: Not a Combination Product 04/20/2020 6 NDC:74743-007-06 60 g in 1 BOTTLE; Type 0: Not a Combination Product 04/20/2020 7 NDC:74743-007-07 50 g in 1 BOTTLE; Type 0: Not a Combination Product 04/20/2020 8 NDC:74743-007-08 30 g in 1 BOTTLE; Type 0: Not a Combination Product 04/20/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 04/20/2020 Labeler - Guangzhou Joy Claire Biological Technology Co., Ltd (418209894) Establishment Name Address ID/FEI Business Operations Guangzhou Joy Claire Biological Technology Co., Ltd 418209894 manufacture(74743-007)