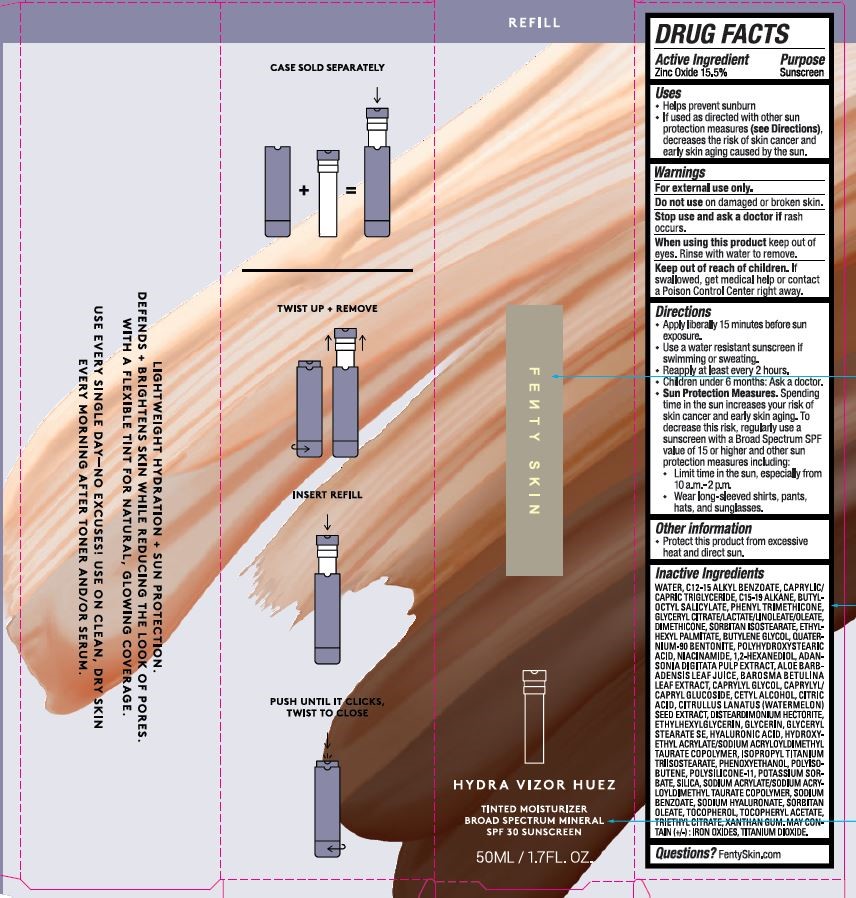
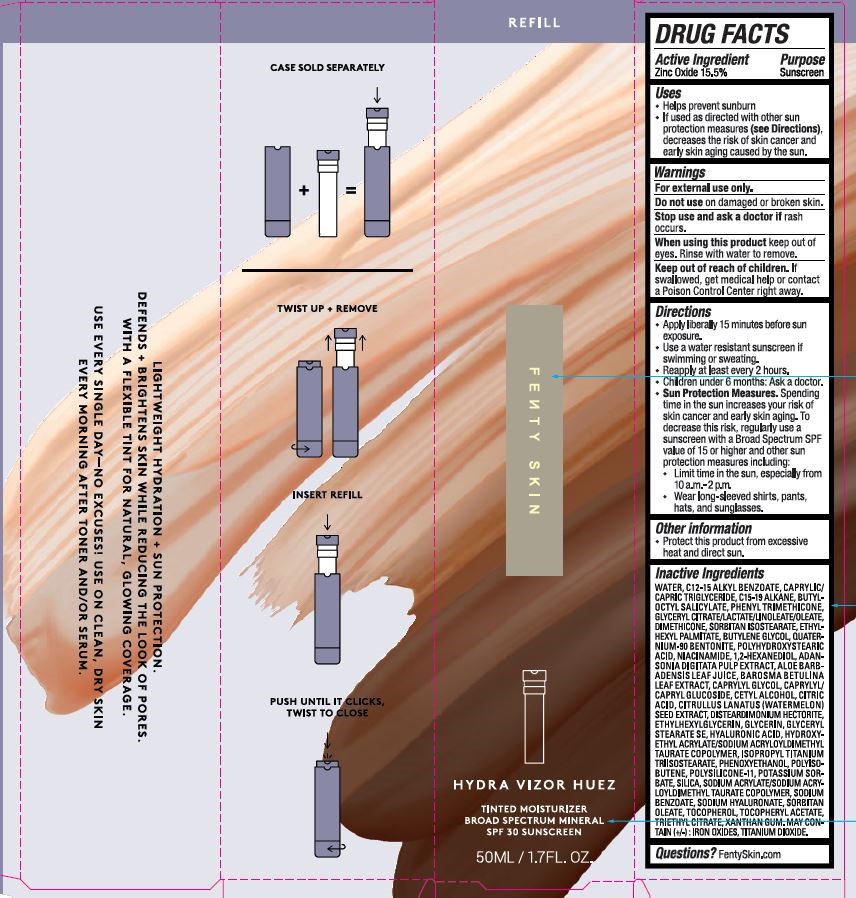
Label: HYDRA VIZOR HUEZ TINTED MOISTURIZER BROAD SPECTRUM SPF 30- zinc oxide lotion
- NDC Code(s): 71499-083-01
- Packager: KENDO HOLDINGS INC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated May 24, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- USES
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
-
DIRECTIONS
DIRECTIONS
APPLY LIBERALLY 15 MINUTES BEFORE SUN EXPOSURE.
USE A WATER RESISTANT SUNSCREEN IF SWIMMING OR SWEATING.
REAPPLY AT LEAST EVERY 2 HOURS.
CHILDREN UNDER 6 MONTHS: ASK A DOCTOR.
SUN PROTECTION MEASURES. SPENDING TIME IN THE SUN INCREASES YOUR RISK OF SKIN CANCER AND EARLY SKIN AGING. TO DECREASE THIS RISK, REGULARLY USE A SUNCREEN WITH A BROAD SPECTRUM SPF VALUE OF 15 OR HIGHER AND OTHER SUN PROTECTION MEASURES INCLUDING:
LIMIT TIME IN THE SUN, ESPECIALLY FROM 10 A.M. - 2 P.M.
WEAR LONG=SLEEVED SHIRTS, PANTS, HATS, AND SUNGLASSES. - OTHER INFORMATION
-
INACTIVE INGREDIENTS
WATER, C12-15 ALKYL BENZOATE, CAPRYLIC/CAPRIC TRIGLYCERIDE, C15-19 ALKANE, BUTYLOCTYL SALICYLATE, PHENYL TRIMETHICONE, GLYCERYL CITRATE/LACTATE/LINOLEATE/OLEATE, DIMETHICONE, SORBITAN ISOSTEARATE, ETHYLHEXYL PALMITATE, BUTYLENE GLYCOL, QUATERNIUM-90 BENTONITE, POLYHYDROXYSTEARIC ACID, NIACINAMIDE, 1,2-HEXANEDIOL, ADANSONIA DIGITATA PULP EXTRACT, ALOE BARBADENSIS LEAF JUICE, BAROSMA BETULINA LEAF EXTRACT, CAPRYLYL GLYCOL, CAPRYLYL/CAPRYL GLUCOSIDE, CETYL ALCOHOL, CITRIC ACID, CITRULLUS LANATUS (WATERMELON) SEED EXTRACT, DISTEARDIMONIUM HECTORITE, ETHYLHEXYLGLYCERIN, GLYCERIN, GLYCERYL STEARATE SE, HYALURONIC ACID, HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER, ISOPROPYL TITANIUM TRIISOSTEARATE, PHENOXYETHANOL, POLYISOBUTENE, POLYSILICONE-11, POTASSIUM SORBATE, SILICA, SODIUM ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER, SODIUM BENZOATE, SODIUM HYALURONATE, SORBITAN OLEATE, TOCOPHEROL, TOCOPHERYL ACETATE, TRIETHYL CITRATE, XANTHAN GUM. MAY CONTAIN (+/-): IRON OXIDES,TITANIUM DIOXIDE.
- QUESTIONS?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
HYDRA VIZOR HUEZ TINTED MOISTURIZER BROAD SPECTRUM SPF 30
zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71499-083 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 15.5 g in 100 mL Inactive Ingredients Ingredient Name Strength BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ISOPROPYL TITANIUM TRIISOSTEARATE (UNII: 949E3KBJ1I) SODIUM ACRYLATE/SODIUM ACRYLOYLDIMETHYLTAURATE COPOLYMER (4000000 MW) (UNII: 1DXE3F3OZX) SODIUM BENZOATE (UNII: OJ245FE5EU) CAPRYLYL/CAPRYL OLIGOGLUCOSIDE (UNII: E00JL9G9K0) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) CETYL ALCOHOL (UNII: 936JST6JCN) WATERMELON SEED (UNII: N364973Y9Q) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) HYALURONATE SODIUM (UNII: YSE9PPT4TH) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) GLYCERYL CITRATE/LACTATE/LINOLEATE/OLEATE (UNII: A5SAE9KW2M) HYALURONIC ACID (UNII: S270N0TRQY) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) XANTHAN GUM (UNII: TTV12P4NEE) PHENOXYETHANOL (UNII: HIE492ZZ3T) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) C15-19 ALKANE (UNII: CI87N1IM01) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) ETHYLHEXYL PALMITATE (UNII: 2865993309) QUATERNIUM-90 BENTONITE (UNII: 97K5YEF88C) NIACINAMIDE (UNII: 25X51I8RD4) ADANSONIA DIGITATA FRUIT PULP (UNII: P65OHK3GHP) BROWN IRON OXIDE (UNII: 1N032N7MFO) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) POLYISOBUTYLENE (1300 MW) (UNII: 241BN7J12Y) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) GLYCERIN (UNII: PDC6A3C0OX) HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER (45000 MPA.S AT 1%) (UNII: 86FQE96TZ4) SORBITAN MONOOLEATE (UNII: 06XEA2VD56) AGATHOSMA BETULINA LEAF (UNII: 369DDH39Z0) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) DIMETHICONE 20 (UNII: H8YMB5QY0D) ALOE VERA LEAF (UNII: ZY81Z83H0X) WATER (UNII: 059QF0KO0R) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71499-083-01 1 in 1 BOX 05/24/2024 1 50 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 05/24/2024 Labeler - KENDO HOLDINGS INC. (078489982) Registrant - KENDO HOLDINGS INC. (078489982)