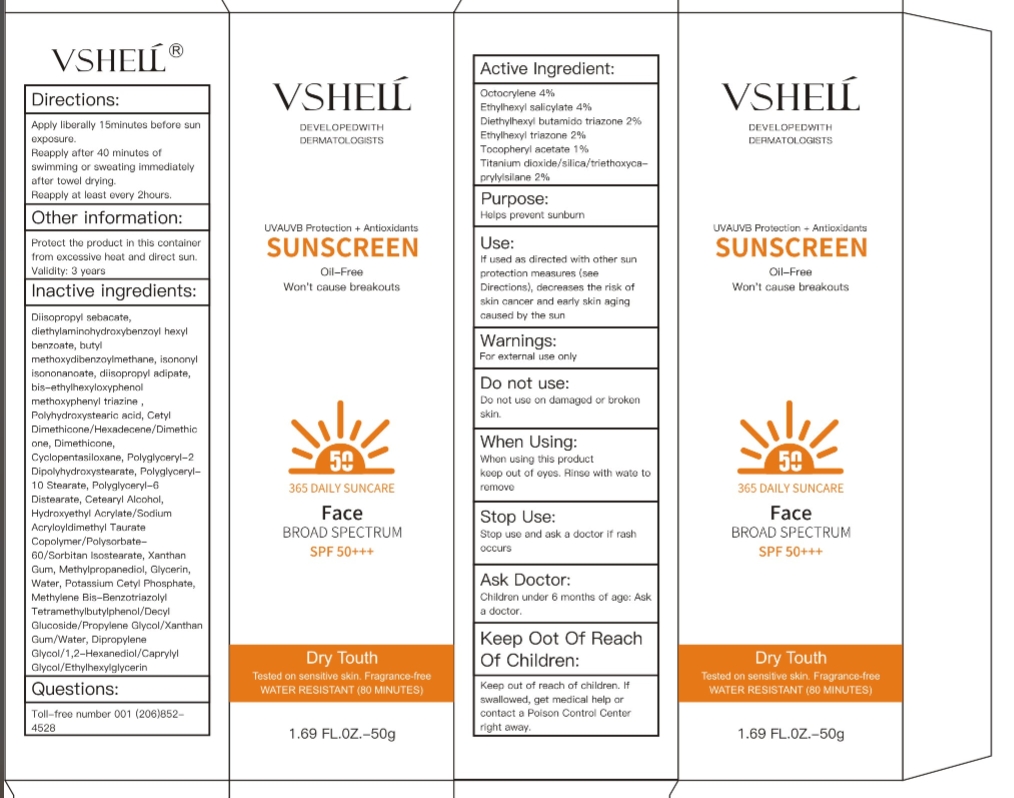
Label: VSHELL SUNSCREEN- sunscreen cream
- NDC Code(s): 84507-001-01
- Packager: Guangdong Miaolian Cosmetics Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Export only
Drug Label Information
Updated July 10, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SUNSCREEN PACKAGE
uses
1.Active Ingredient: Octocrylene 4%
Ethylhexyl salicylate 4%
Diethylhexyl butamido triazone 2%
Ethylhexyl triazone 2%
Tocopheryl acetate 1%
Titanium dioxide/silica/triethoxycaprylylsilane 2%
2.Purpose: Helps prevent sunburn
3.Use: If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
4.Warnings: For external use only
5.Do not use: Do not use on damaged or broken skin.
6.When Using : When using this product
keep out of eyes. Rinse with wate to remove
Stop use and ask doctor if rash occurs.
7.Stop Use : Stop use and ask a doctor if rash occurs
8.Ask Doctor: Children under 6 months of age: Ask a doctor.
9.Keep Oot Of Reach Of Children
: Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
10.Directions: Apply liberally 15minutes before sun exposure.
Reapply after 40 minutes of swimming or sweating immediately after towel drying.
Reapply at least every 2hours.
11.Other information: Protect the product in this container from excessive heat and direct sun.
Validity: 3 years
12.Inactive ingredients: Diisopropyl sebacate, diethylaminohydroxybenzoyl hexyl benzoate, butyl methoxydibenzoylmethane, isononyl isononanoate, diisopropyl adipate, bis-ethylhexyloxyphenol methoxyphenyl triazine , Polyhydroxystearic acid, Cetyl Dimethicone/Hexadecene/Dimethicone, Dimethicone, Cyclopentasiloxane, Polyglyceryl-2 Dipolyhydroxystearate, Polyglyceryl-10 Stearate, Polyglyceryl-6 Distearate, Cetearyl Alcohol, Hydroxyethyl Acrylate/Sodium Acryloyldimethyl Taurate Copolymer/Polysorbate-60/Sorbitan Isostearate, Xanthan Gum, Methylpropanediol, Glycerin, Water, Potassium Cetyl Phosphate, Methylene Bis-Benzotriazolyl Tetramethylbutylphenol/Decyl Glucoside/Propylene Glycol/Xanthan Gum/Water, Dipropylene Glycol/1,2-Hexanediol/Caprylyl Glycol/Ethylhexylglycerin
13.Questions: Toll-free number 001 (206)852-4528 -
INGREDIENTS AND APPEARANCE
VSHELL SUNSCREEN
sunscreen creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84507-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISCOTRIZINOL (UNII: 2UTZ0QC864) (ISCOTRIZINOL - UNII:2UTZ0QC864) ISCOTRIZINOL 2 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 4 g in 100 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 2 g in 100 mL TOCOTRIENOLS (UNII: KP2MW85SSQ) (TOCOTRIENOLS - UNII:KP2MW85SSQ) TOCOTRIENOLS 1 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 4 g in 100 mL ETHYLHEXYL TRIAZONE (UNII: XQN8R9SAK4) (ETHYLHEXYL TRIAZONE - UNII:XQN8R9SAK4) ETHYLHEXYL TRIAZONE 2 g in 100 mL Inactive Ingredients Ingredient Name Strength DIISOPROPYL SEBACATE (UNII: J8T3X564IH) METHYLPROPANEDIOL (UNII: N8F53B3R4R) XANTHAN GUM (UNII: TTV12P4NEE) POLYGLYCERYL-6 DISTEARATE (UNII: Z35I17EQOP) HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER (45000 MPA.S AT 1%) (UNII: 86FQE96TZ4) WATER (UNII: 059QF0KO0R) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) GLYCERIN (UNII: PDC6A3C0OX) DIMETHICONE (UNII: 92RU3N3Y1O) BEMOTRIZINOL (UNII: PWZ1720CBH) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) POLYGLYCERYL-2 DIPOLYHYDROXYSTEARATE (UNII: 9229XJ4V12) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) DIETHYLAMINO HYDROXYBENZOYL HEXYL BENZOATE (UNII: ANQ870JD20) AVOBENZONE (UNII: G63QQF2NOX) DIISOPROPYL ADIPATE (UNII: P7E6YFV72X) 1-HEXADECENE (UNII: 97T015M2UX) POLYGLYCERYL-10 STEARATE (UNII: 90TF85HH91) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) DIPROPYLENE GLYCOL (UNII: E107L85C40) BISOCTRIZOLE (UNII: 8NT850T0YS) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84507-001-01 50 mL in 1 BOTTLE; Type 0: Not a Combination Product 07/13/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 07/13/2024 Labeler - Guangdong Miaolian Cosmetics Co., Ltd. (707598469) Establishment Name Address ID/FEI Business Operations Guangdong Miaolian Cosmetics Co., Ltd. 707598469 manufacture(84507-001)