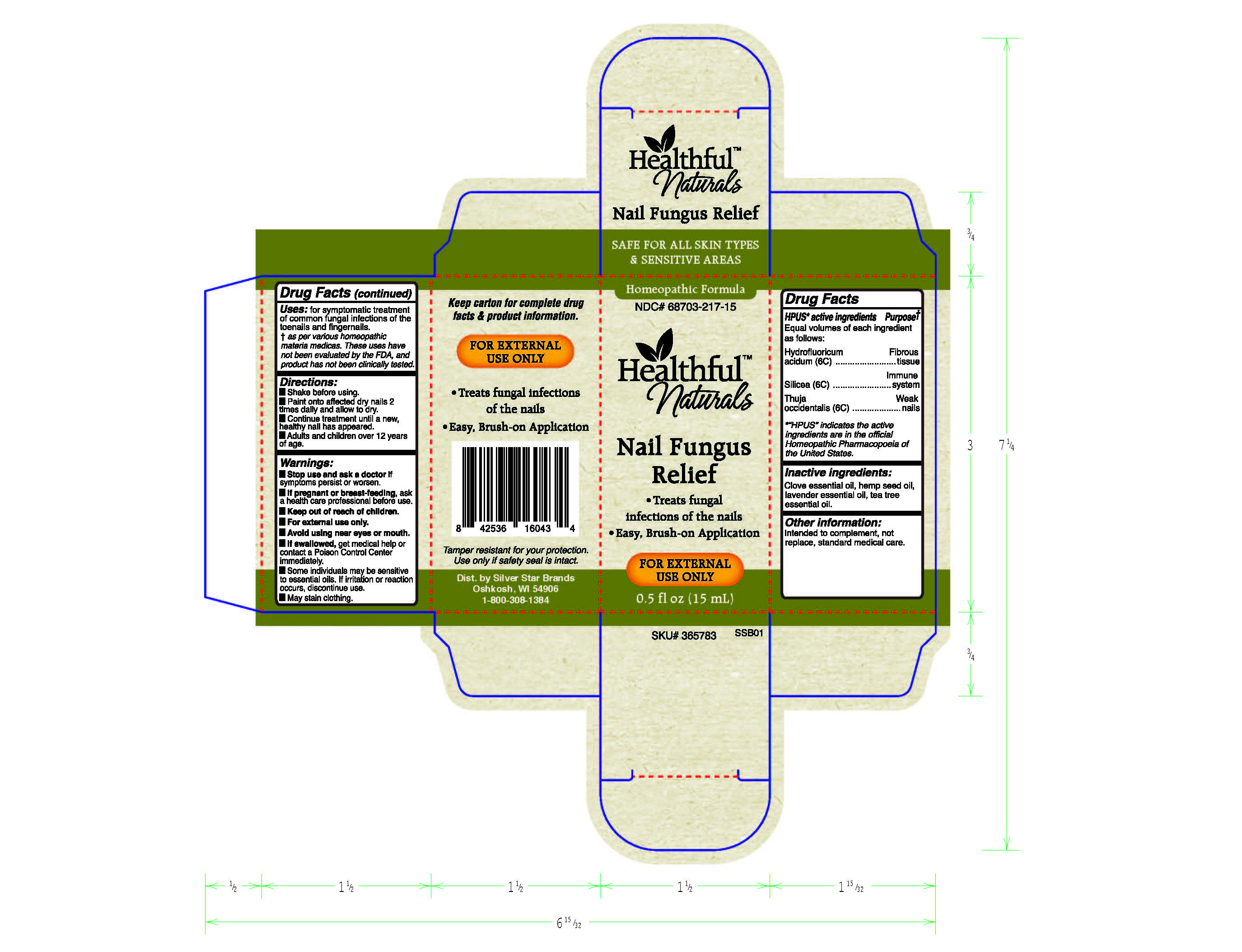
Label: GOUT CALM- antimonium crudum, berberis vulgaris, lithium carbonicum spray
- NDC Code(s): 68703-218-02
- Packager: Silver Star Brands
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated March 4, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- HPUS* Active ingredients
- Uses+:
-
Warnings
Warnings: If symptoms persist or worsen, discontinue use immediately and consult a healthcare professional.
If pregnant or breast-feeding, ask a health care professional before use.
Keep out of reach of children.
For external use only.
Avoid using near eyes or mouth.
If swallowed, get medical help or contact a Poison Control Center immediately.Some individuals may be sensitive to essential oils. If irritation or reaction occurs, discontinue use.
- Directions
- OTHER SAFETY INFORMATION
- Inactive ingredients
- KEEP OUT OF REACH OF CHILDREN
- PREGNANCY OR BREAST FEEDING
- PURPOSE
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GOUT CALM
antimonium crudum, berberis vulgaris, lithium carbonicum sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68703-218 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ANTIMONY TRISULFIDE (UNII: F79059A38U) (ANTIMONY TRISULFIDE - UNII:F79059A38U) ANTIMONY TRISULFIDE 6 [hp_C] in 59 mL BERBERIS VULGARIS FRUIT (UNII: 6XEF22AHC3) (BERBERIS VULGARIS FRUIT - UNII:6XEF22AHC3) BERBERIS VULGARIS FRUIT 30 [hp_C] in 59 mL LITHIUM CARBONATE (UNII: 2BMD2GNA4V) (LITHIUM CATION - UNII:8H8Z5UER66) LITHIUM CARBONATE 6 [hp_C] in 59 mL Inactive Ingredients Ingredient Name Strength BLACK PEPPER OIL (UNII: U17J84S19Z) GRAPE SEED OIL (UNII: 930MLC8XGG) BASIL OIL (UNII: Z129UMU8LE) CELERY SEED OIL (UNII: 8MBL58728K) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68703-218-02 59 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 01/14/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 01/14/2019 Labeler - Silver Star Brands (006070379) Registrant - Silver Star Brands (006070379) Establishment Name Address ID/FEI Business Operations Vitality Works 137752817 manufacture(68703-218)