Label: WANG PROM GREEN BALM BY WANG PROM- wang prom wang prom green balm by venture go llc patch
- NDC Code(s): 84212-333-50
- Packager: AMBIENCE FAMILY INC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated May 7, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DOSAGE & ADMINISTRATION
- WARNINGS
- WARNINGS
- INACTIVE INGREDIENT
-
INDICATIONS & USAGE
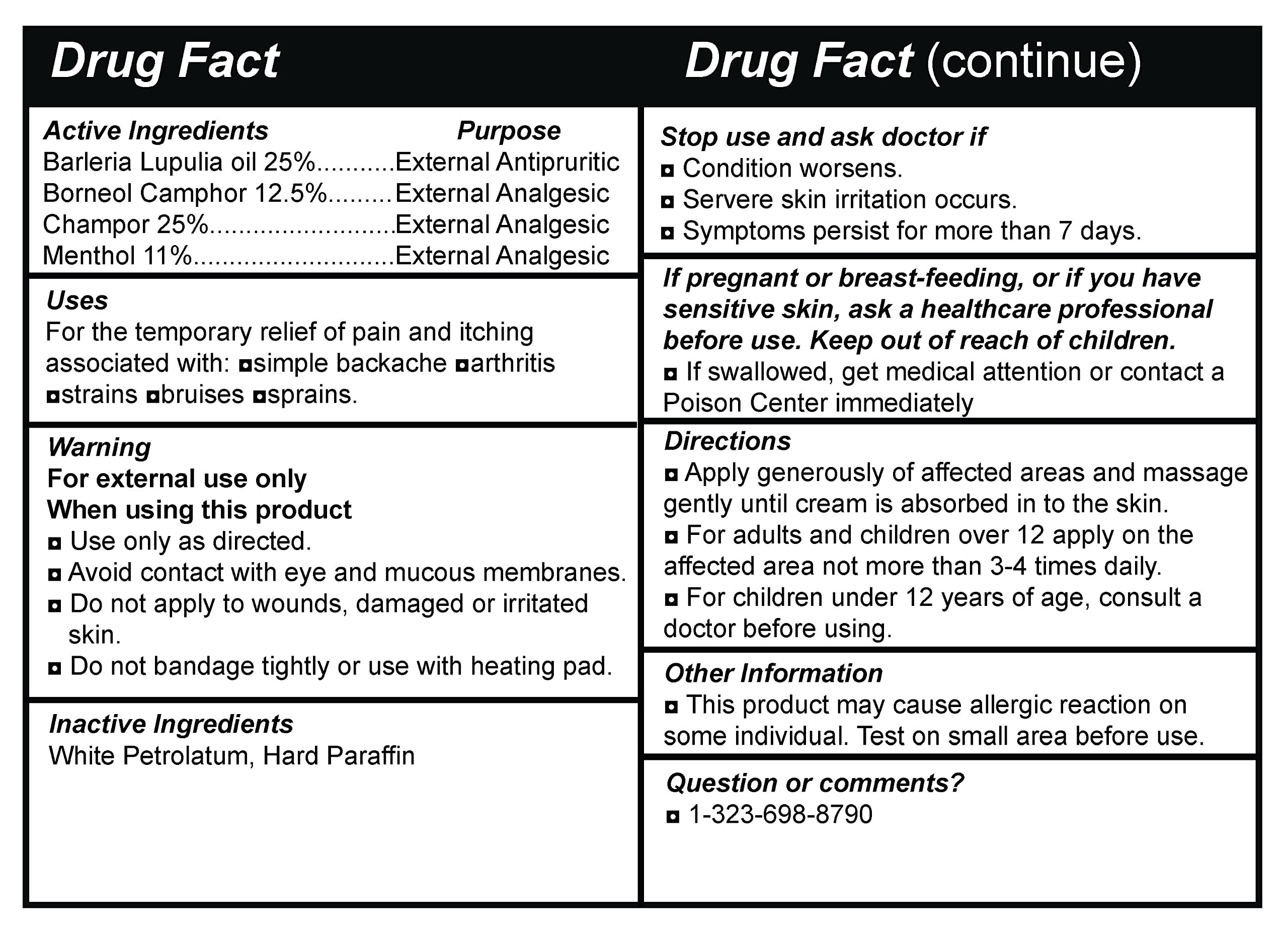
Uses For the temporary relief of pain and itching associated with: asimple backache aarthritis nstrains abruises nsprains.
Directions a Apply generously of affected areas and massage gently until cream is absorbed in to the skin. a For adults and children over 12 apply on the affected area not more than 3-4 times daily. a For children under 12 years of age, consult a doctor before using.
Other Information a This product may cause allergic reaction on some individual. Test on small area before use.
- KEEP OUT OF REACH OF CHILDREN
- PURPOSE
- ACTIVE INGREDIENT
-
PRINCIPAL DISPLAY PANEL
 Drug Fact Drug Fact (continue)
Drug Fact Drug Fact (continue)
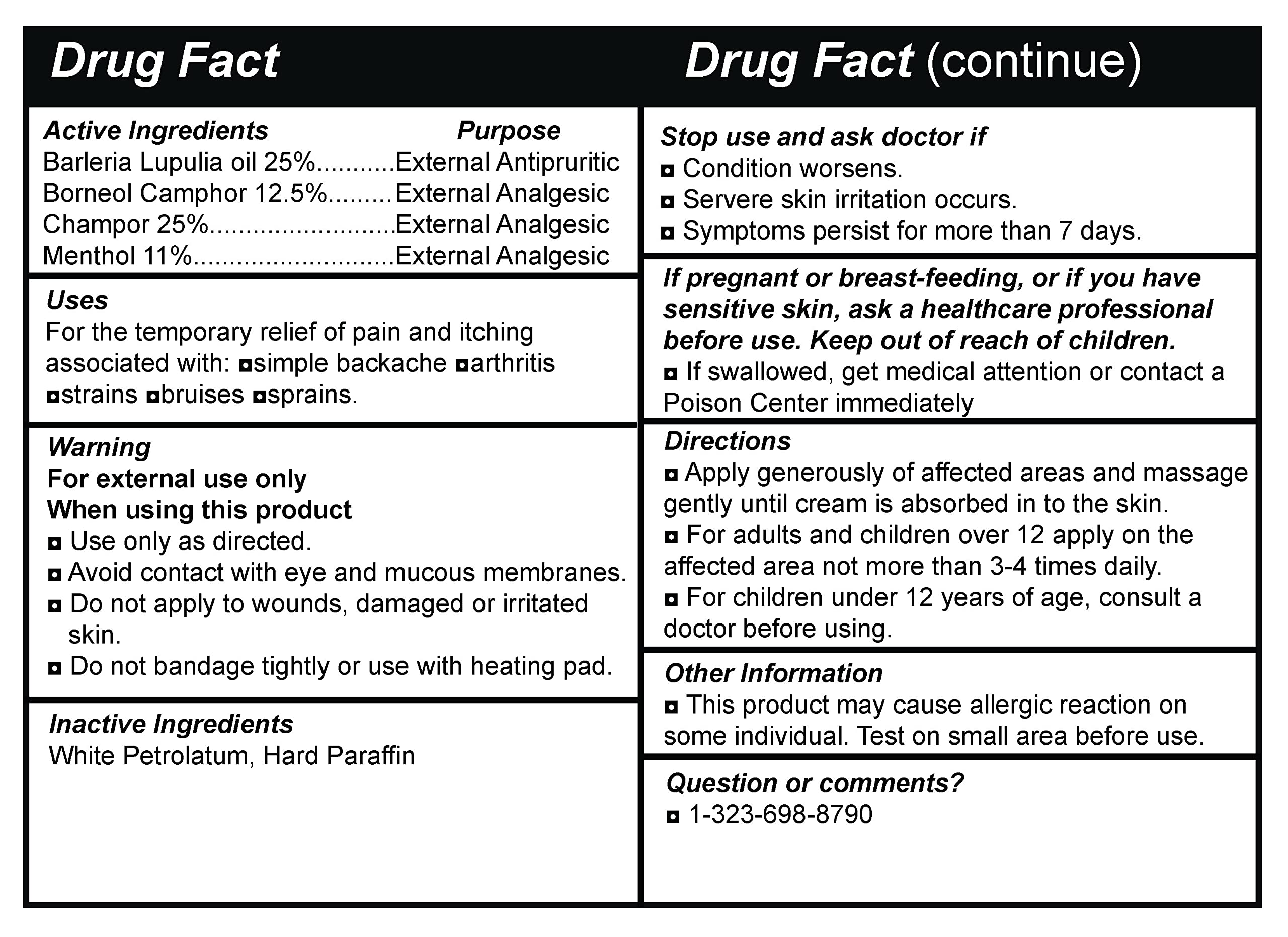
Active Ingredients Purpose Barleria Lupulia oil 25% External Antipruritic Borneo! Camphor 12.5% External Analgesic Champor 25% External Analgesic Menthol 11% External Analgesic
Uses For the temporary relief of pain and itching associated with: asimple backache aarthritis nstrains abruises nsprains.
Warning For external use only When using this product a Use only as directed. a Avoid contact with eye and mucous membranes. a Do not apply to wounds, damaged or irritated skin. a Do not bandage tightly or use with heating pad.
Inactive Ingredients White Petrolatum, Hard Paraffin
Stop use and ask doctor if a Condition worsens. Servere skin irritation occurs. a Symptoms persist for more than 7 days.
If pregnant or breast-feeding, or if you have sensitive skin, ask a healthcare professional before use. Keep out of reach of children. a If swallowed, get medical attention or contact a Poison Center immediately
Directions a Apply generously of affected areas and massage gently until cream is absorbed in to the skin. a For adults and children over 12 apply on the affected area not more than 3-4 times daily. a For children under 12 years of age, consult a doctor before using.
Other Information a This product may cause allergic reaction on some individual. Test on small area before use.
Question or comments? a 1-323-698-8790 -
INGREDIENTS AND APPEARANCE
WANG PROM GREEN BALM BY WANG PROM
wang prom wang prom green balm by venture go llc patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84212-333 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 5.5 g in 100 g BORNEOL (UNII: M89NIB437X) (BORNEOL - UNII:M89NIB437X) BORNEOL 6.25 g in 100 g CINNAMOMUM CAMPHORA LEAF (UNII: A0TKE4ZO0S) (CINNAMOMUM CAMPHORA LEAF - UNII:A0TKE4ZO0S) CINNAMOMUM CAMPHORA LEAF 12.5 g in 100 g BARLERIA LUPULINA WHOLE (UNII: 8J6B3YN1PM) (BARLERIA LUPULINA WHOLE - UNII:8J6B3YN1PM) BARLERIA LUPULINA WHOLE 12.5 g in 100 g Inactive Ingredients Ingredient Name Strength PARAFFIN (UNII: I9O0E3H2ZE) WHITE PETROLATUM (UNII: B6E5W8RQJ4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84212-333-50 50 g in 1 BOX; Type 0: Not a Combination Product 05/01/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 05/01/2024 Labeler - AMBIENCE FAMILY INC (084561491) Registrant - Venture Go LLC (118882840)