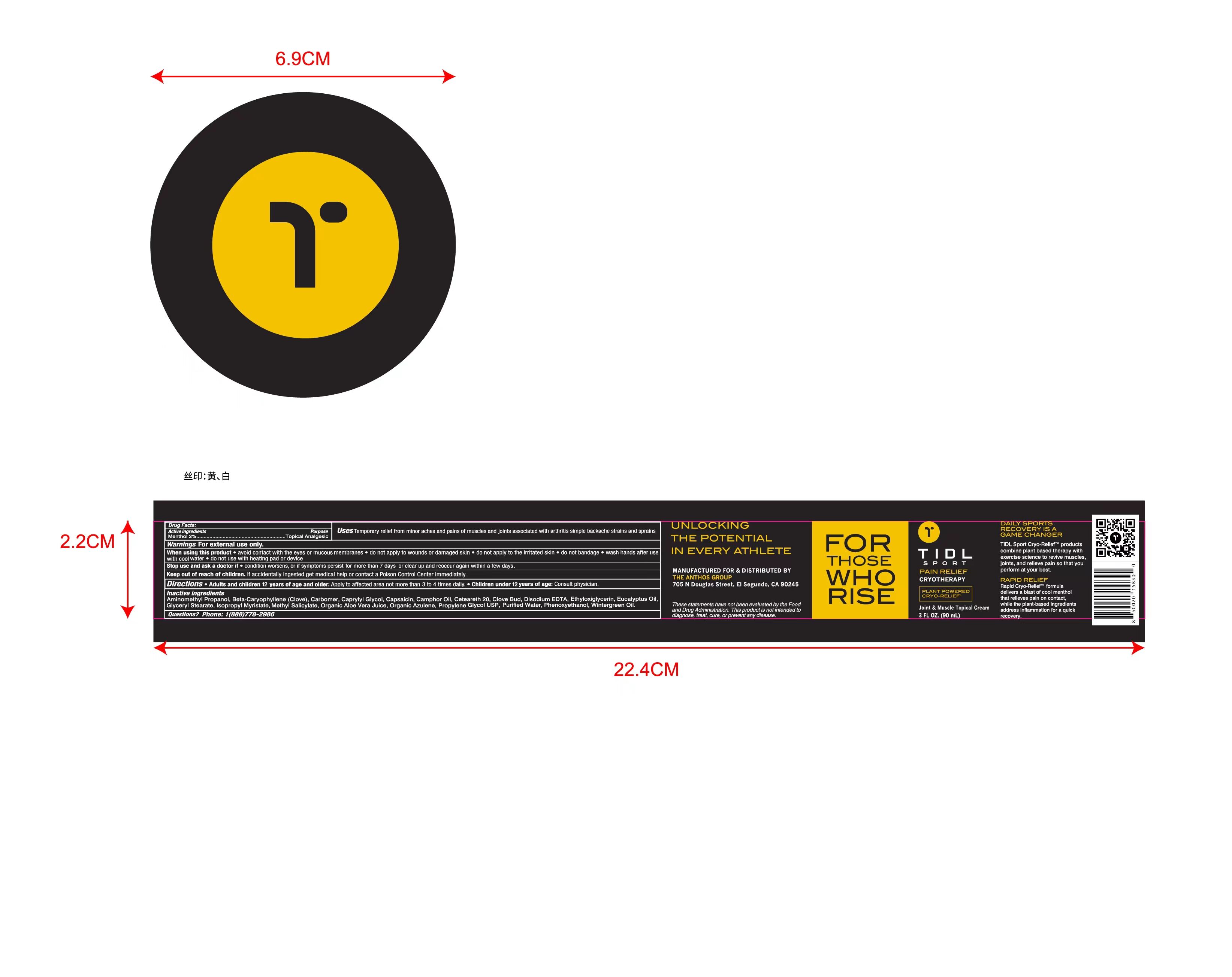
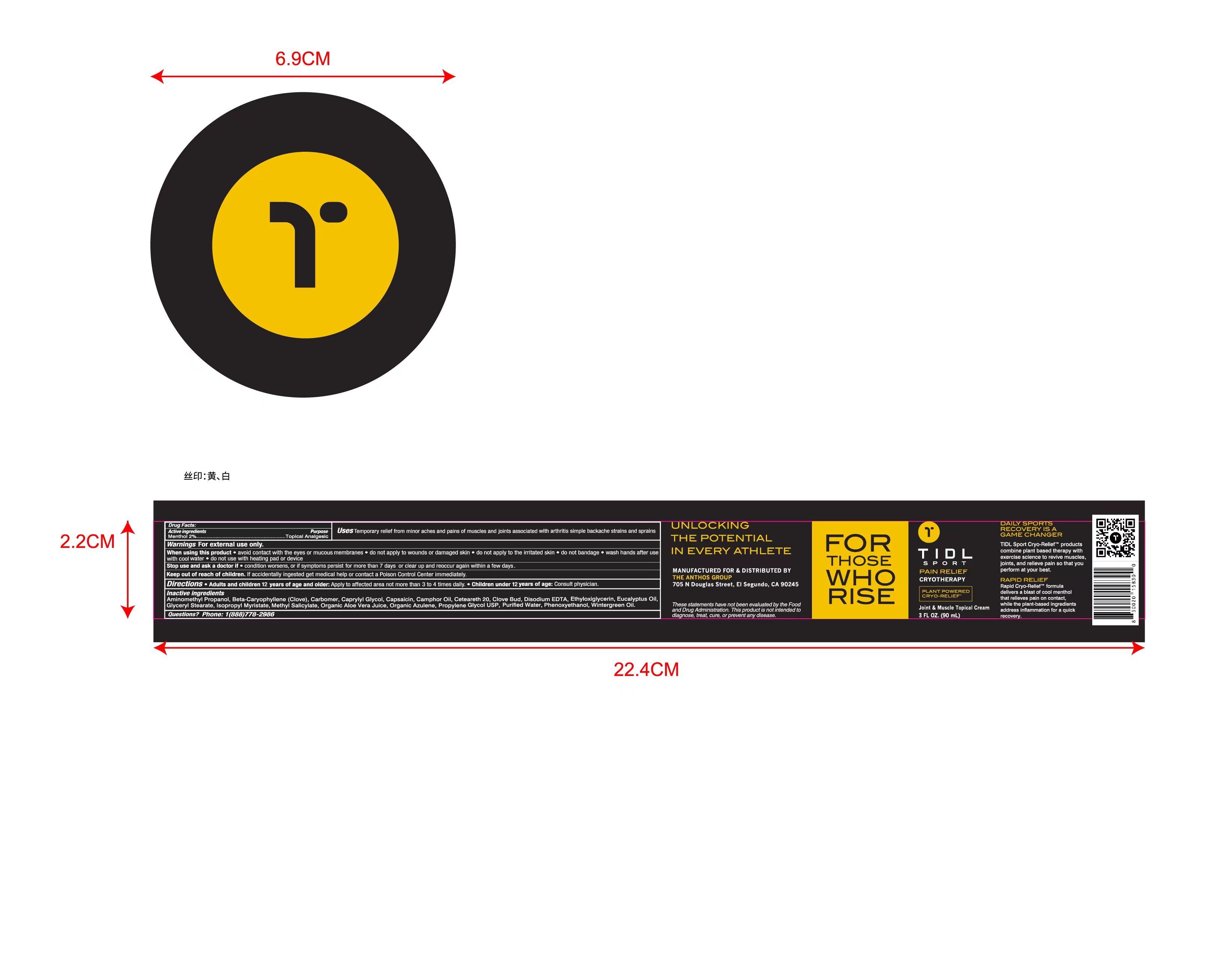
Label: TIDL SPORT CRYOTHERAPY JOINT MUSCLE cream
- NDC Code(s): 79740-008-01, 79740-008-02
- Packager: ANTHOS GROUP, INC, THE
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 20, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient(s)
- Purpose
- Use
- Warnings
- Do not use
- STOP USE
- WHEN USING
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Other information
-
Inactive ingredients
Purified Water, Propylene Glycol USP, Organic Aloe Vera Juice, Isopropyl Myristate, Ceteareth 20, Methyl Salicylate, Eucalyptus Oil, Wintergreen Oil, Camphor Oil, Clove Bud, Beta-Caryophyllene(Clove), Glyceryl Stearate, Phenoxyethanol, Caprylyl Glycol, Ethyloxiglycerin, Disodium EDTA, Capsaicin, Organic Azulene, Carbomer, Aminomethyl Propanol
- Package Label - Principal Display Panel
-
INGREDIENTS AND APPEARANCE
TIDL SPORT CRYOTHERAPY JOINT MUSCLE
tidl sport cryotherapy joint muscle creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:79740-008 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 2 g in 100 mL Inactive Ingredients Ingredient Name Strength EDETATE DISODIUM (UNII: 7FLD91C86K) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) 1,2-PROPANEDIOL, 1-BENZOATE (UNII: K4K90ZQ89N) ALOE VERA LEAF (UNII: ZY81Z83H0X) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) METHYL SALICYLATE (UNII: LAV5U5022Y) EUCALYPTUS OIL (UNII: 2R04ONI662) WATER (UNII: 059QF0KO0R) CLOVE (UNII: K48IKT5321) CARYOPHYLLENE (UNII: BHW853AU9H) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) PHENOXYETHANOL (UNII: HIE492ZZ3T) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CAMPHOR LEAF OIL (UNII: 51D0RGY52V) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) AMINOMETHYLPROPANOL (UNII: LU49E6626Q) CAPSAICIN (UNII: S07O44R1ZM) AZULENE (UNII: 82R6M9MGLP) POLYOXYL 20 CETOSTEARYL ETHER (UNII: YRC528SWUY) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:79740-008-01 90 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/20/2022 2 NDC:79740-008-02 90 mL in 1 BOX; Type 0: Not a Combination Product 01/20/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 01/20/2022 Labeler - ANTHOS GROUP, INC, THE (117511051) Establishment Name Address ID/FEI Business Operations ANTHOS GROUP, INC, THE 117511051 manufacture(79740-008)