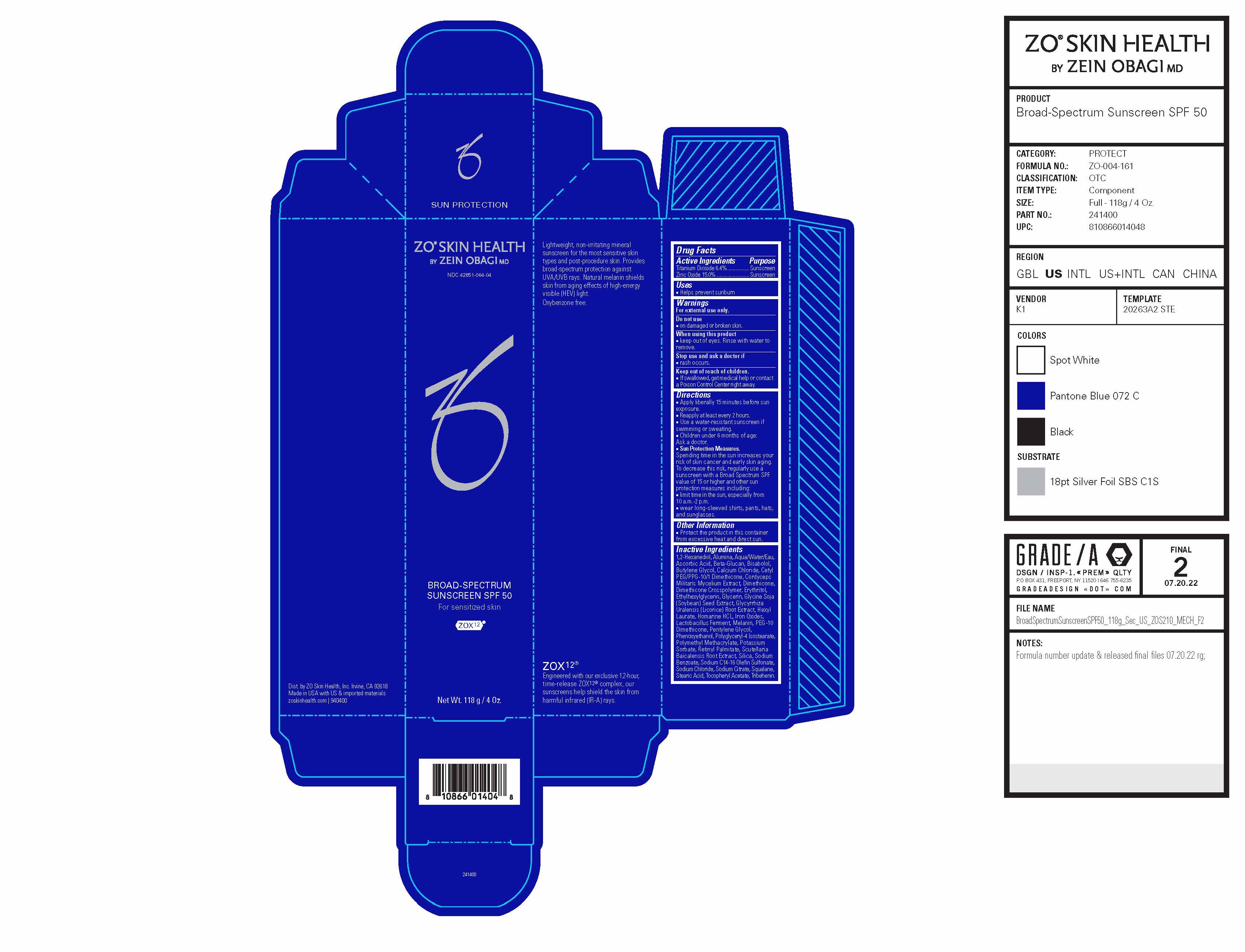
Label: ZO SKIN HEALTH BROAD-SPECTRUM SUNSCREEN SPF 50- titanium dioxide, zinc oxide cream
- NDC Code(s): 42851-064-04
- Packager: ZO Skin Health, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated September 7, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Drug Facts
Active Ingredients
Active Ingredient Purpose Titanium Dioxide 6.4% Sunscreen Zinc Oxide 15.0% Sunscreen Keep out of reach of children.
- If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Apply liberally 15 minutes before sun exposure.
- Reapply at least every 2 hours.
- Use a water-resistant sunscreen if swimming or sweating.
- Children under 6 months of age: Ask a doctor.
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including: ■ limit time in the sun, especially from 10 a.m.-2 p.m. ■ wear long-sleeved shirts, pants, hats,
and sunglasses.
Inactive Ingredients
1,2-Hexanediol, Alumina, Aqua/Water/Eau, Ascorbic Acid, Beta-Glucan, Bisabolol, Butylene Glycol, Calcium Chloride, Cetyl PEG/PPG-10/1 Dimethicone, Cordyceps Militaris Mycelium Extract, Dimethicone, Dimethicone Crosspolymer, Erythritol, Ethylhexylglycerin, Glycerin, Glycine Soja (Soybean) Seed Extract, Glycyrrhiza Uralensis (Licorice) Root Extract, Hexyl Laurate, Homarine HCL, Iron Oxides, Lactobacillus Ferment, Melanin, PEG-10 Dimethicone, Pentylene Glycol, Phenoxyethanol, Polyglyceryl-4 Isostearate, Polymethyl Methacrylate, Potassium Sorbate, Retinyl Palmitate, Scutellaria Baicalensis Root Extract, Silica, Sodium Benzoate, Sodium C14-16 Olefin Sulfonate, Sodium Chloride, Sodium Citrate, Squalane, Stearic Acid, Tocopheryl Acetate, Tribehenin.
-
Outer Package Label
ZO® Skin Health
by ZEIN OBAGI MD
NDC 42851-064-04
BROAD-SPECTRUM
SUNSCREEN SPF 50
For sensitized skinZOX 12®
Lightweight, non-irritating mineral sunscreen for the most sensitive skin types and post-procedure skin. Provides broad-spectrum protection against UVA/UVB rays. Natural melanin shields skin from aging effects of high-energy visible (HEV) light. Oxybenzone free.
ZOX 12®
Engineered with our exclusive 12-hour, time-release ZOX12® complex, our sunscreens help shield the skin from harmful infrared (IR-A) rays.
Dist. by ZO Skin Health, Inc. Irvine, CA 92618
Made in USA with US & imported materials
zoskinhealth.com -
INGREDIENTS AND APPEARANCE
ZO SKIN HEALTH BROAD-SPECTRUM SUNSCREEN SPF 50
titanium dioxide, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:42851-064 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 7.552 mg in 118 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 17.7 mg in 118 g Inactive Ingredients Ingredient Name Strength PHENOXYETHANOL (UNII: HIE492ZZ3T) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) ALUMINUM OXIDE (UNII: LMI26O6933) WATER (UNII: 059QF0KO0R) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) ERYTHRITOL (UNII: RA96B954X6) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) HEXYL LAURATE (UNII: 4CG9F9W01Q) GLYCYRRHIZA URALENSIS ROOT (UNII: 42B5YD8F0K) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) GLYCERIN (UNII: PDC6A3C0OX) ASCORBIC ACID (UNII: PQ6CK8PD0R) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) DIMETHICONE (UNII: 92RU3N3Y1O) HOMARINE HYDROCHLORIDE (UNII: 8866LNG61N) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) SOLANUM ANOMALUM WHOLE (UNII: 7J95G4E8OY) SODIUM C14-16 OLEFIN SULFONATE (UNII: O9W3D3YF5U) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM CITRATE (UNII: 1Q73Q2JULR) SODIUM BENZOATE (UNII: OJ245FE5EU) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) FERRIC OXIDE RED (UNII: 1K09F3G675) SCUTELLARIA LATERIFLORA TOP (UNII: C6CNB75R61) STEARIC ACID (UNII: 4ELV7Z65AP) SQUALANE (UNII: GW89575KF9) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) LEVOMENOL (UNII: 24WE03BX2T) PENTYLENE GLYCOL (UNII: 50C1307PZG) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) TRIBEHENIN (UNII: 8OC9U7TQZ0) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:42851-064-04 1 in 1 CARTON 08/15/2022 1 118 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 08/15/2022 Labeler - ZO Skin Health, Inc. (826468527) Establishment Name Address ID/FEI Business Operations LIFETECH RESOURCES LLC 622559110 manufacture(42851-064)