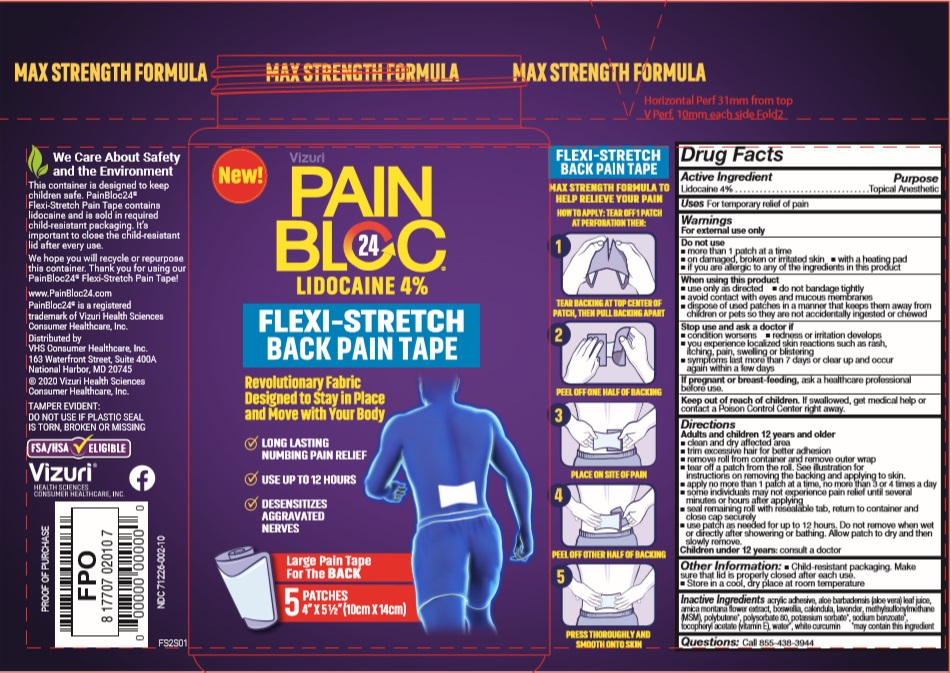
Label: PAINBLOC24 FLEXI-STRETCH PAIN TAPE- lidocaine patch
-
Contains inactivated NDC Code(s)
NDC Code(s): 71226-002-01, 71226-002-09, 71226-002-10, 71226-002-17 - Packager: VIZURI HEALTH SCIENCES LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated October 1, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- Uses
-
Warnings
For external use only
Do not use:
- more than 3 strips at a time
- on damaged, broken or irritated skin
- with a heating pad
- if you are allergic to any of the ingredients in this product
When using this product use only as directed:
- do not bandage tightly
- avoid contact with eyes and mucous membranes
- dispose of used strips in a manner that keeps them away from children or pets so they are not accidentally ingested or chewed
Stop use and ask a doctor if:- condition worsens
- redness or irritation develops
- you experience localized skin reactions such as rash, itching, pain, swelling or blistering
- symptoms last more than 7 days or clear up and occur again within a few days
If pregnant or breast-feeding, ask a healthcare professional before use.
- Keep out of reach of children.
-
Directions
Adults and Children 12 years and older
- Clean and dry affected area
- Trim excessive hair for better adhesion
- Remove roll from container and remove outer wrap
- Tear off 1 or 2 strips from roll. See illustration for instructions on removing the backing and applying to skin.
- Apply no more than 3 strips at a time, no more than 2 times a day
- Some individuals may not experience pain relief until several minutes or hours after applying
- Seal remaining roll with resealable tab, return to container and close cap securely
- Use strip as needed for up to 12 hours. Do not remove when wet or directly after showering or bathing. Allow strip to dry and then remove.
Children under 12 years: consult a doctor
- Inactive Ingredients
- PRINCIPAL DISPLAY PANEL
- Product label
- Product label
-
INGREDIENTS AND APPEARANCE
PAINBLOC24 FLEXI-STRETCH PAIN TAPE
lidocaine patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71226-002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 4 g Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) BOSWELLIA FREREANA WHOLE (UNII: 795ST8633Q) CALENDULA ARVENSIS LEAF (UNII: 3U3U118F2L) LAVANDULA ANGUSTIFOLIA SUBSP. ANGUSTIFOLIA FLOWER (UNII: 19AH1RAF4M) DIMETHYL SULFONE (UNII: 9H4PO4Z4FT) POLYSORBATE 80 (UNII: 6OZP39ZG8H) POLYBUTENE (1400 MW) (UNII: 1NA5AO9GH7) WATER (UNII: 059QF0KO0R) PEPPERMINT OIL (UNII: AV092KU4JH) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) CURCUMIN (UNII: IT942ZTH98) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71226-002-01 10 in 1 CANISTER; Type 0: Not a Combination Product 06/25/2020 09/30/2021 2 NDC:71226-002-10 5 in 1 CANISTER; Type 0: Not a Combination Product 06/25/2020 3 NDC:71226-002-17 10 in 1 CARTON; Type 0: Not a Combination Product 09/30/2021 4 NDC:71226-002-09 10 in 1 CARTON; Type 0: Not a Combination Product 06/25/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 06/25/2020 Labeler - VIZURI HEALTH SCIENCES LLC (052129499)