Label: ANTIGEN COMPONENT- cov-2 pres dtm antigen injection, emulsion
-
Contains inactivated NDC Code(s)
NDC Code(s): 49281-618-20, 49281-618-78 - Packager: Sanofi Pasteur Inc.
- Category: VACCINE LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
Drug Label Information
Updated November 9, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 10 Dose Vial Label
NDC 49281-618-78
Vial 1 of 2
ANTIGEN COMPONENT
for Sanofi Pasteur COVID-19 Vaccine, AdjuvantedAfter mixing, emulsion for intramuscular injection
For use under Emergency Use Authorization.
NOT TO BE USED ALONE.
Add 1 vial of AS03 Adjuvant Component to form the Vaccine.
Multiple Dose Vial (after mixing, contains 10 doses of 0.5 mL). After
mixing, hold at 23°C to 27°C (73°F to 80°F). Discard after 6 hours.
Refer to FDA-authorized Fact Sheet for mixing instructions.
No Preservative
Record date and time of mixing:
Manufactured by: Sanofi Pasteur Inc.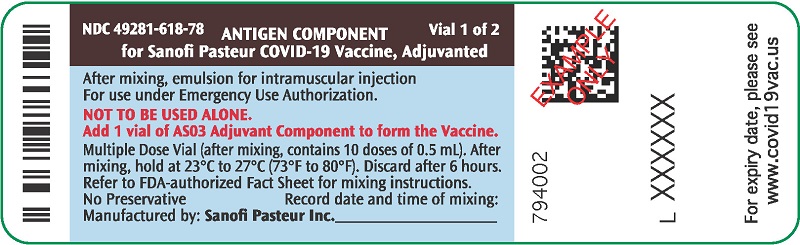
-
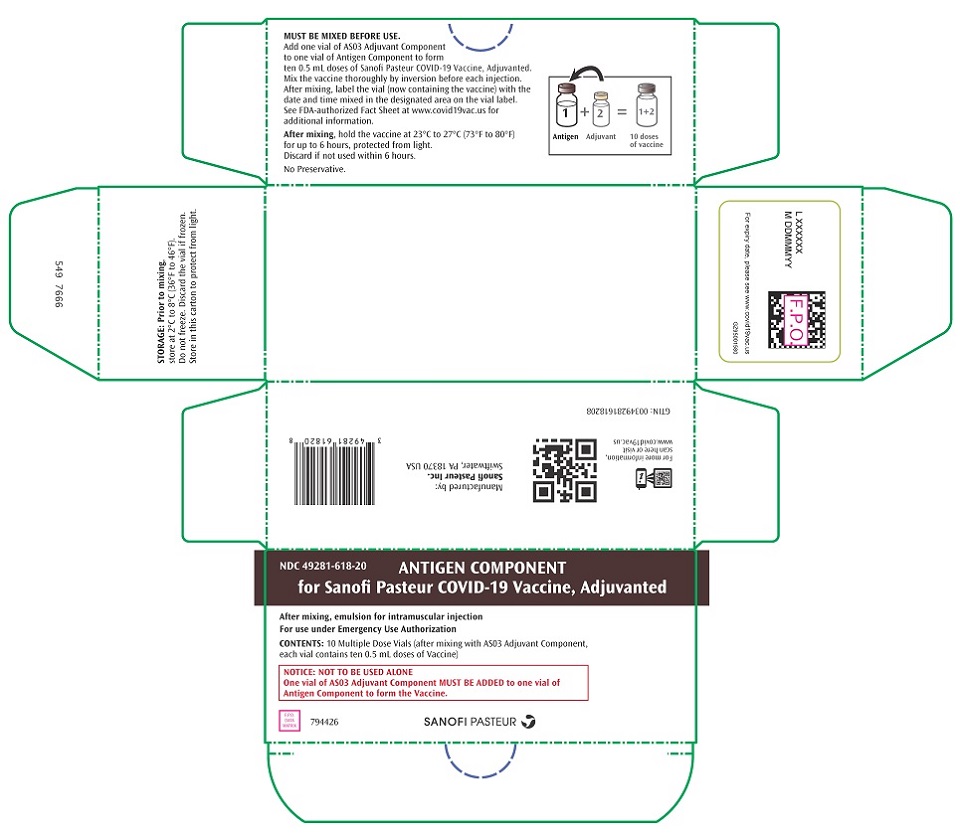
PRINCIPAL DISPLAY PANEL - 10 Vial Carton
NDC 49281-618-20
ANTIGEN COMPONENT
for Sanofi Pasteur COVID-19 Vaccine, AdjuvantedAfter mixing, emulsion for intramuscular injection
For use under Emergency Use AuthorizationCONTENTS: 10 Multiple Dose Vials (after mixing with AS03 Adjuvant Component,
each vial contains ten 0.5 mL doses of Vaccine)NOTICE: NOT TO BE USED ALONE
One vial of AS03 Adjuvant Component MUST BE ADDED to one vial of
Antigen Component to form the Vaccine.794426
SANOFI PASTEUR
-
INGREDIENTS AND APPEARANCE
ANTIGEN COMPONENT
cov-2 pres dtm antigen injection, emulsionProduct Information Product Type VACCINE Item Code (Source) NDC:49281-618 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength COV-2 PRES DTM ANTIGEN (UNII: 76SQ8FDG7I) (COV-2 PRES DTM ANTIGEN - UNII:76SQ8FDG7I) COV-2 PRES DTM ANTIGEN 50 ug in 2.5 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) 22 mg in 2.5 mL SODIUM PHOSPHATE, DIBASIC, DODECAHYDRATE (UNII: E1W4N241FO) 6.5 mg in 2.5 mL SODIUM PHOSPHATE, MONOBASIC, MONOHYDRATE (UNII: 593YOG76RN) 0.975 mg in 2.5 mL POLYSORBATE 20 (UNII: 7T1F30V5YH) 13.75 mg in 2.5 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49281-618-20 10 in 1 CARTON 1 NDC:49281-618-78 2.5 mL in 1 VIAL, MULTI-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED DRUG OTHER 11/10/2020 Labeler - Sanofi Pasteur Inc. (086723285) Establishment Name Address ID/FEI Business Operations Sanofi Pasteur Inc. 086723285 MANUFACTURE(49281-618)


