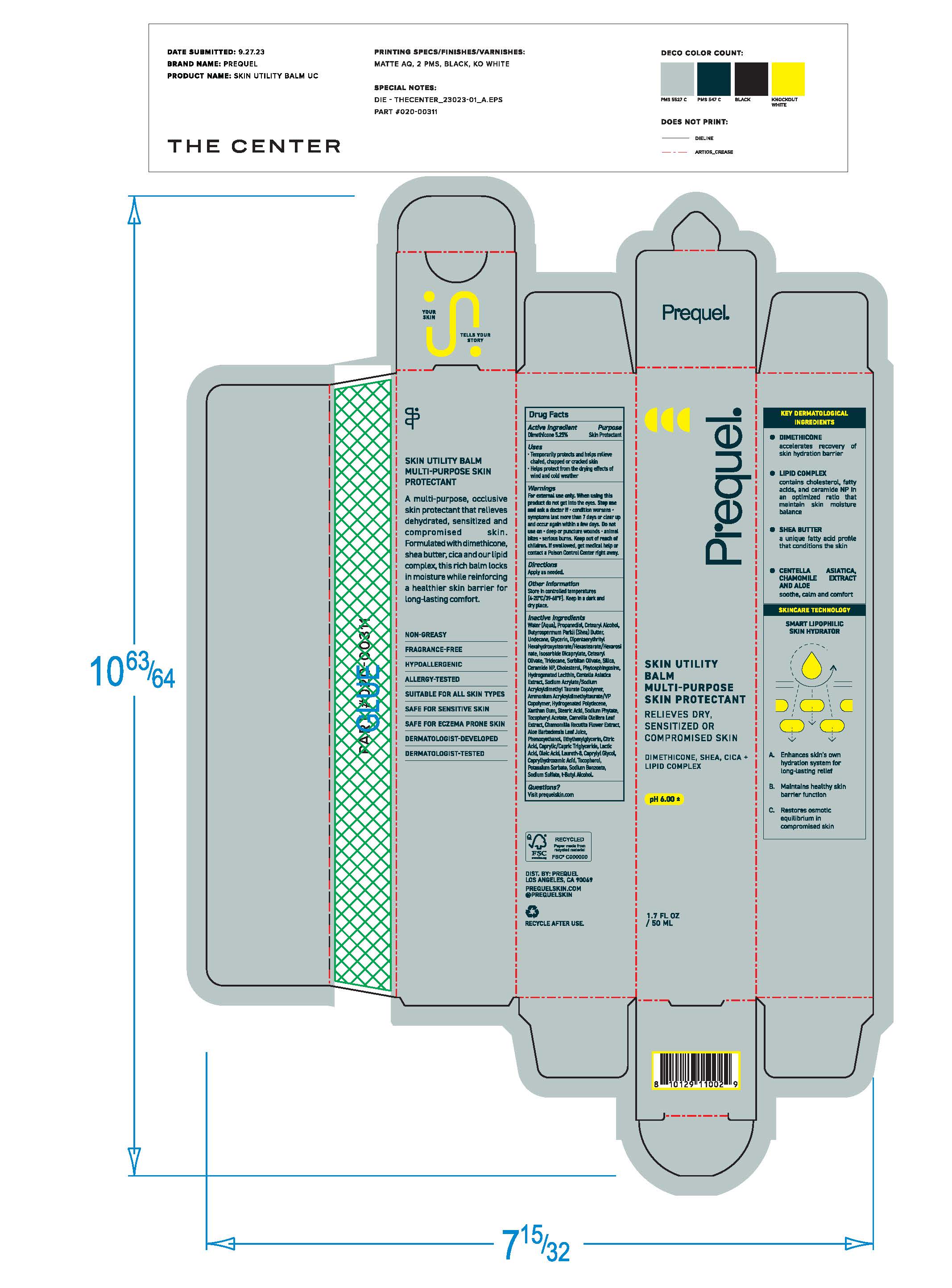
Label: PREQUEL SKIN UTILITY BALM MULTI-PURPOSE SKIN PROTECTANT- skin protectant balm ointment
- NDC Code(s): 82800-060-01
- Packager: The Center Brands, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 4, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Purpose
- Active Ingredients
- Uses
- Warnings
- Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
- Directions
-
Inactive ingredients
Water (Aqua), Propanediol, Cetearyl Alcohol, Butyrospermum Parkii (Shea) Butter, Undecane, Glycerin, Dipentaerythryityl Hexahydrostearate/Hexastearate/Hexarosinate, Isosorbate Dicaprylate, Cetearyl Olivate, Tridecane, Sorbitan Olivate, Silica, Ceramide NP, Cholesterol, Phytospingosine, Hydrogentaed Lecithin, Centella Asiatica Extract, Sodium Acrylate/Sodium Acryloyldimethyl Taurate Copolymer, Ammonium Acryloyldimethyltuarate/VP Copolymer, Hydrogenated Polydecene, Xanthan Gum, Stearic Acid, Sodium Phytate, Tocopheryl Acetate, Camomilla Recutita Flower Extract, Aloe Barbadensis Leaf Juice, Phenoxyethanol, Ethylhexylglycerin, Citric Acid, Caprylic/Capric Trigylceride, Lactic Acid, Oleic Acid, Laureth-8, Caprylyl Glycol, Caprylhydroxamic Acid, Tocopherol, Potassium Sorbate, Sodium Benzoate, Sodium Sulfate, t-Butyl Acohol
- Other Information
- Questions?
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
PREQUEL SKIN UTILITY BALM MULTI-PURPOSE SKIN PROTECTANT
skin protectant balm ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82800-060 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIMETHICONE (UNII: 92RU3N3Y1O) (DIMETHICONE - UNII:92RU3N3Y1O) DIMETHICONE 5.25 mg in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PROPANEDIOL (UNII: 5965N8W85T) BUTYROSPERMUM PARKII (SHEA) BUTTER UNSAPONIFIABLES (UNII: 0C9AC7D6XU) UNDECANE (UNII: JV0QT00NUE) DIPENTAERYTHRITYL HEXASTEARATE (UNII: WTF09990PK) GLYCERIN (UNII: PDC6A3C0OX) ISOSORBIDE DICAPRYLATE (UNII: 0IK29C4889) CETEARYL OLIVATE (UNII: 58B69Q84JO) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) PHENOXYETHANOL (UNII: HIE492ZZ3T) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) CERAMIDE NP (UNII: 4370DF050B) CHOLESTEROL (UNII: 97C5T2UQ7J) PHYTOSPHINGOSINE (UNII: GIN46U9Q2Q) HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) CENTELLA ASIATICA (UNII: 7M867G6T1U) SODIUM ACRYLATE/SODIUM ACRYLOYLDIMETHYLTAURATE COPOLYMER (4000000 MW) (UNII: 1DXE3F3OZX) AMMONIUM ACRYLOYLDIMETHYLTAURATE/VP COPOLYMER (UNII: W59H9296ZG) HYDROGENATED POLYDECENE TYPE I (UNII: U333RI6EB7) XANTHAN GUM (UNII: TTV12P4NEE) STEARIC ACID (UNII: 4ELV7Z65AP) HEXASODIUM PHYTATE (UNII: ZBX50UG81V) CAMELLIA OLEIFERA LEAF (UNII: 5077EL0C60) CHAMOMILE FLOWER OIL (UNII: 60F80Z61A9) ALOE VERA LEAF (UNII: ZY81Z83H0X) CITRIC ACID ACETATE (UNII: DSO12WL7AU) LACTIC ACID (UNII: 33X04XA5AT) OLEIC ACID (UNII: 2UMI9U37CP) LAURETH-8 (UNII: QU7U88D04I) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CAPRYLHYDROXAMIC ACID (UNII: UPY805K99W) TOCOPHEROL (UNII: R0ZB2556P8) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM SULFATE (UNII: 0YPR65R21J) TERT-BUTYL ACETATE (UNII: 76N22HKP3X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82800-060-01 1 in 1 PACKAGE 10/30/2023 1 50 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 10/30/2023 Labeler - The Center Brands, LLC (076228814)