Label: NA- volanesorsen powder
- NDC Code(s): 72126-001-00
- Packager: Akcea Therapeutics, Inc.
- Category: BULK INGREDIENT
- DEA Schedule: None
- Marketing Status: Export only
Drug Label Information
Updated May 24, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
NA
volanesorsen powderProduct Information Product Type BULK INGREDIENT Item Code (Source) NDC:72126-001 Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength VOLANESORSEN SODIUM (UNII: 6JON30SLDT) (VOLANESORSEN - UNII:2O4BE0K238) VOLANESORSEN SODIUM 1 kg in 1 kg Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72126-001-00 11 kg in 1 BOTTLE, PLASTIC 05/03/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 05/03/2019 Labeler - Akcea Therapeutics, Inc. (079911419) Registrant - Akcea Therapeutics, Inc. (079911419)


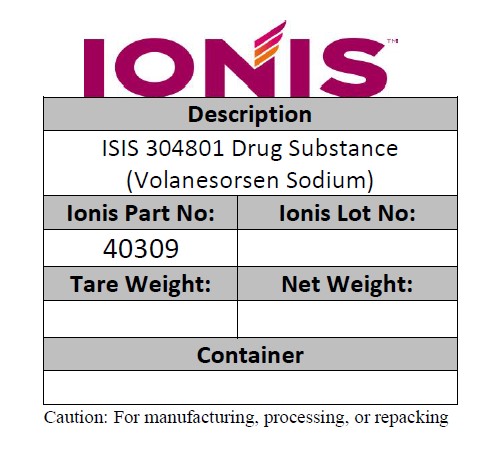 Description of ISIS 304801 Drug Substance (Volanesorsen Sodium)
Description of ISIS 304801 Drug Substance (Volanesorsen Sodium)