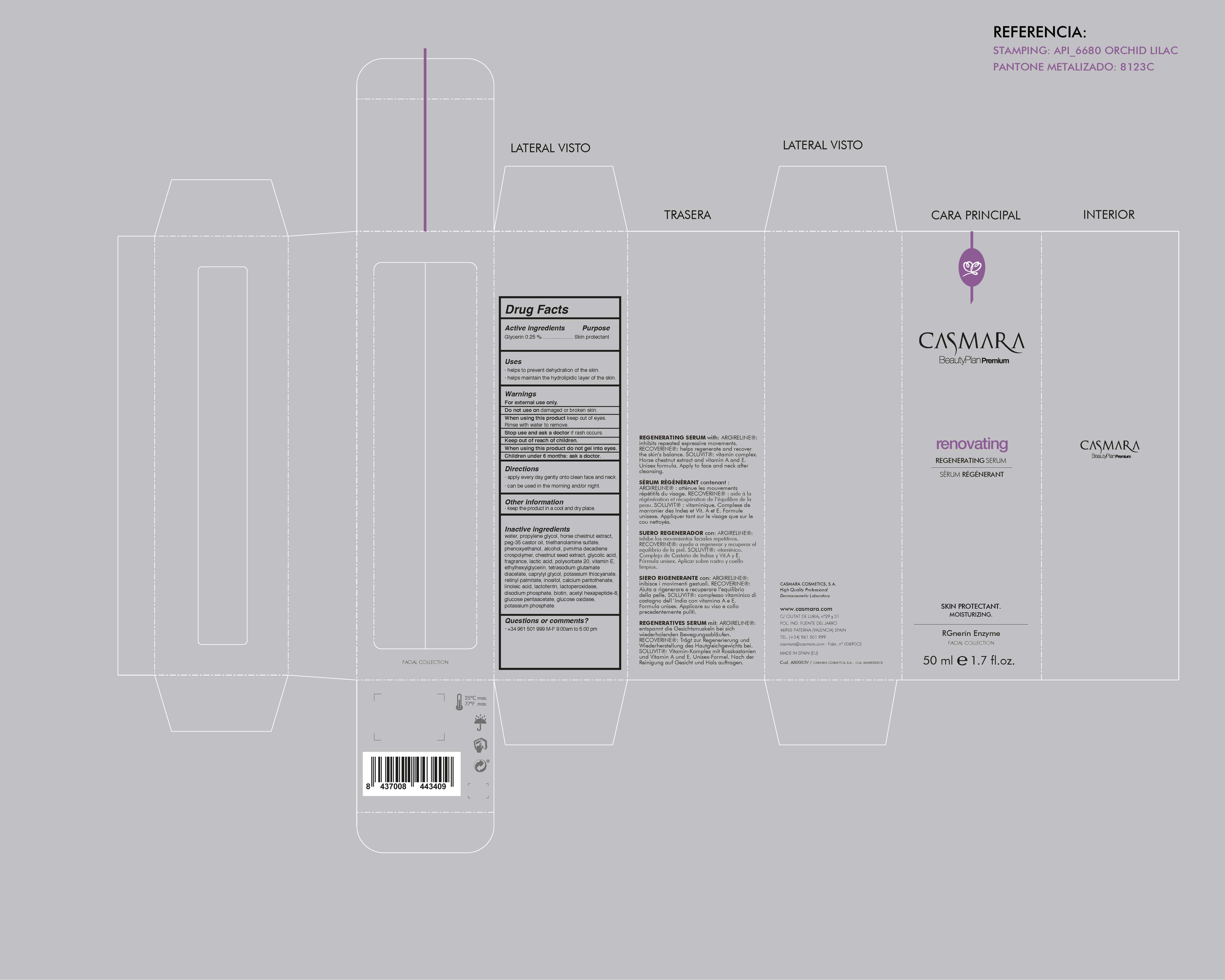
Label: RENOVATING REGENERATING SERUM- glycerin cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 20151-068-01, 20151-068-02 - Packager: Casmara Cosmetics, SA
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated November 1, 2016
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- WARNINGS
- ASK DOCTOR
- KEEP OUT OF REACH OF CHILDREN
- Questions or Comments?
- OTHER SAFETY INFORMATION
- Directions
- DOSAGE & ADMINISTRATION
- Uses
-
INACTIVE INGREDIENT
water, propylene glycol, horse chestnut extract, PEG-25 castor oil, triethanolamine, phenoxyethanol, alcohol, PVM/MA Decadiene crospolymer, chestnut seed extract, glycolic acid, fragrance, lactic acid, polysorbate 20, tocopherol, ethylhexylglycerin, tetrasodium glutamate diacetate, caprylyl glycol, potassium thiocyanate, retinyl palmitate, inositol, calcium pantothenate, linoleic acid, lactoferrin, lactoperoxidase, disodium phosphate, biotin, acetyl hexapeptide-8, glucose pentaacetate, glucose oxidase, potassium phosphate
- PURPOSE
- Active Ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
RENOVATING REGENERATING SERUM
glycerin creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:20151-068 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GLYCERIN (UNII: PDC6A3C0OX) (GLYCERIN - UNII:PDC6A3C0OX) GLYCERIN 0.25 mg in 1 mL Inactive Ingredients Ingredient Name Strength POLYOXYL 35 CASTOR OIL (UNII: 6D4M1DAL6O) 1.875 mg in 1 mL BUTYL ESTER OF METHYL VINYL ETHER-MALEIC ANHYDRIDE COPOLYMER (125 KD) (UNII: 389H2R62BD) 0.8 mg in 1 mL HORSE CHESTNUT (UNII: 3C18L6RJAZ) 0.3 mg in 1 mL POLYSORBATE 20 (UNII: 7T1F30V5YH) 0.15 mg in 1 mL LINOLEIC ACID (UNII: 9KJL21T0QJ) 0.027 mg in 1 mL LACTOPEROXIDASE BOVINE (UNII: 01UIN5OC49) 0.015 mg in 1 mL BIOTIN (UNII: 6SO6U10H04) 0.005 mg in 1 mL CALCIUM PANTOTHENATE, (-)- (UNII: 6IY177DF2V) 0.027 mg in 1 mL POTASSIUM THIOCYANATE (UNII: TM7213864A) 0.037 mg in 1 mL LACTOFERRIN, BOVINE (UNII: KG21X1090A) 0.025 mg in 1 mL GLUCOSE PENTAACETATE (UNII: 3040885R4N) 0.0035 mg in 1 mL GLUCOSE OXIDASE (UNII: 0T8392U5N1) 0.001 mg in 1 mL VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) 0.027 mg in 1 mL SPANISH CHESTNUT (UNII: 2MT5XMR2YW) 1.875 mg in 1 mL GLYCOLIC ACID (UNII: 0WT12SX38S) 0.21 mg in 1 mL CAPRYLYL GLYCOL (UNII: 00YIU5438U) 0.05 mg in 1 mL ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) 0.106 mg in 1 mL WATER (UNII: 059QF0KO0R) 72.75 mg in 1 mL PHENOXYETHANOL (UNII: HIE492ZZ3T) 0.915 mg in 1 mL TETRASODIUM GLUTAMATE DIACETATE (UNII: 5EHL50I4MY) 0.094 mg in 1 mL TOCOPHEROL (UNII: R0ZB2556P8) 0.15 mg in 1 mL PERFLUNAFENE (UNII: 54A06VV62N) 0.2 mg in 1 mL POTASSIUM PHOSPHATE, MONOBASIC (UNII: 4J9FJ0HL51) 0.001 mg in 1 mL TROLAMINE (UNII: 9O3K93S3TK) 1 mg in 1 mL DIPROPYLENE GLYCOL (UNII: E107L85C40) 18 mg in 1 mL SODIUM PHOSPHATE (UNII: SE337SVY37) 0.0115 mg in 1 mL INOSITOL (UNII: 4L6452S749) 0.0275 mg in 1 mL ALCOHOL (UNII: 3K9958V90M) 0.875 mg in 1 mL ACETYL HEXAPEPTIDE-8 (UNII: L4EL31FWIL) 0.005 mg in 1 mL LACTIC ACID (UNII: 33X04XA5AT) 0.18 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:20151-068-02 1 in 1 BOTTLE, DISPENSING 05/13/2016 1 NDC:20151-068-01 50 mL in 1 BOTTLE, DISPENSING; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part347 05/13/2016 Labeler - Casmara Cosmetics, SA (464973544) Registrant - Casmara Cosmetics, SA (464973544) Establishment Name Address ID/FEI Business Operations Casmara Cosmetics, SA 464973544 manufacture(20151-068)