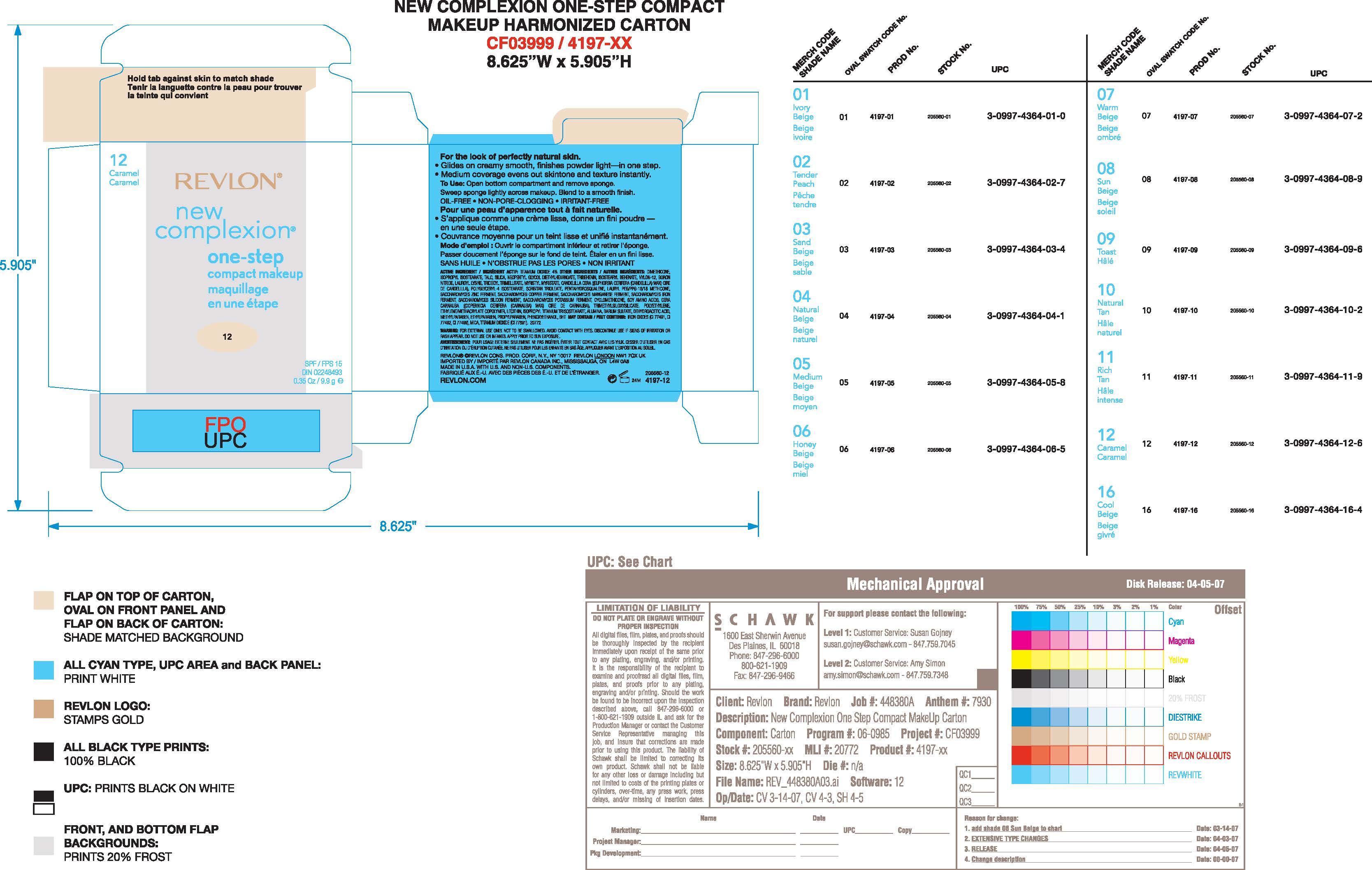
Label: REVLON NEW COMPLEXION ONE-STEP PERFECT MAKE-UP- titanium dioxide make-up paste
- NDC Code(s): 10967-606-35
- Packager: Revlon Consumer Products Corp
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients:
- Uses:
-
Warnings:
- For external use only
- Do notuse on damaged or broken skin
- When using this product: Keep out of eyes. Rinse with water to remove.
- Stop use and ask a doctor if a rash occurs
- Keep out of reach of children. If product is swallowed, get medical help or contact a Poison Control center right away
Directions:
- Apply liberally 15 minutes before sun exposure
- Use a water resistant sunscreen if swimming or sweating
- Reapply at least every 2 hours
- Children under 6 months: Ask a doctor.
Sun Protection Measures:
Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- Limit time in the sun, especially from 10 am - 2 pm
- Wear long-sleeved shirts, pants, hats and sunglasses.
-
Inactive Ingredients:
DIMETHICONE,ISOPROPYL,ISOSTEARATE,TALC,SILICA,NEOPENTYL,GLYCOL,DIETHYLHEXANOATE,TRIBEHENIN,ISOSTEARYL,BEHENATE,NYLON-12,BORON,NITRIDE,LAUROYL,LYSINE,TRIDECYL,TRIMELLITATE,MYRISTYL,MYRISTATE,CANDELILLA,CERA,((EUPHORBIA,CERIFERA,(CANDELILLA)WAX),CIRE,DE,CANDELILLA,POLYGLYCERYL-4,ISOSTEARATE,SORBITAN,TRIOLEATE,PENTAHYDROSQUALENE,LAURYL,PEG/PPG-18/18,METHICONE,SACCHAROMYCES,ZINC,FERMENT,SACCHAROMYCES,COPPER,FERMENT,SACCHAROMYCES,MANGANESE,FERMENT,SACCHAROMYCES,IRON,FERMENT,SACCHAROMYCES,SILICON,FERMENT,SACCHAROMYCES,POTASSIUM,FERMENT,CYCLOMETHICONE,SOY,AMINO,ACIDS,CERA,CARNAUBA,((COPERNICIA,CERIFERA,(CARNAUBA),WAX),CIRE,DE,CARNAUBA,TRIMETHYLSILOXYSILICATE,POLYETHYLENE,ETHYLENE/METHACRYLATE,COPOLYMER,LECITHIN,ISOPROPYL,TITANIUM,TRIISOSTEARATE,ALUMINA,BARIUM,SULFATE,DEHYDROACETIC,ACID,METHYLPARABEN,ETHYLPARABEN,PROPYLPARABEN,PHENOXYETHANOL,BHT
May Contain: Mica, Titanium Dioxide (Ci 77891), Iron Oxides (CI 77491, 77492, 77499)
- DOSAGE & ADMINISTRATION
- INDICATIONS & USAGE
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- Principal Display Panel:
-
INGREDIENTS AND APPEARANCE
REVLON NEW COMPLEXION ONE-STEP PERFECT MAKE-UP
titanium dioxide make-up pasteProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10967-606 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 4 mg in 1 mL Inactive Ingredients Ingredient Name Strength DIMETHICONE (UNII: 92RU3N3Y1O) PHENOXYETHANOL (UNII: HIE492ZZ3T) METHYLPARABEN (UNII: A2I8C7HI9T) ETHYLPARABEN (UNII: 14255EXE39) TRIMETHYLSILOXYSILICATE (M/Q 0.6-0.8) (UNII: 5041RX63GN) TRIBEHENIN (UNII: 8OC9U7TQZ0) NYLON-12 (UNII: 446U8J075B) ALUMINUM OXIDE (UNII: LMI26O6933) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CYCLOMETHICONE 6 (UNII: XHK3U310BA) ISOPROPYL ISOSTEARATE (UNII: C67IXB9Y7T) TALC (UNII: 7SEV7J4R1U) NEOPENTYL GLYCOL DIETHYLHEXANOATE (UNII: U68ZV6W62C) ISOSTEARYL BEHENATE (UNII: NA95U012OW) BORON NITRIDE (UNII: 2U4T60A6YD) LAUROYL LYSINE (UNII: 113171Q70B) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) MYRISTYL MYRISTATE (UNII: 4042ZC00DY) CANDELILLA WAX (UNII: WL0328HX19) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) SORBITAN TRIOLEATE (UNII: QE6F49RPJ1) 2,10,15,19,23-PENTAHYDROSQUALENE (UNII: 6IWZ426YD6) LAURYL PEG/PPG-18/18 METHICONE (UNII: ZJ5S27D9NX) AMINO ACIDS, SOY (UNII: NWB9514AZM) CARNAUBA WAX (UNII: R12CBM0EIZ) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) ISOPROPYL TITANIUM TRIISOSTEARATE (UNII: 949E3KBJ1I) BARIUM SULFATE (UNII: 25BB7EKE2E) DEHYDROACETIC ACID (UNII: 2KAG279R6R) PROPYLPARABEN (UNII: Z8IX2SC1OH) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10967-606-35 9.9 mL in 1 CONTAINER; Type 0: Not a Combination Product 04/01/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/01/2014 Labeler - Revlon Consumer Products Corp (788820165) Establishment Name Address ID/FEI Business Operations REVLON, INC. 809725570 manufacture(10967-606)