Label: ARIZONA SUN PRICKLY PEAR LIP BALM SPF 15- sunscreen lip balm lipstick
- NDC Code(s): 61973-400-10
- Packager: Arizona Sun Products
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 23, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
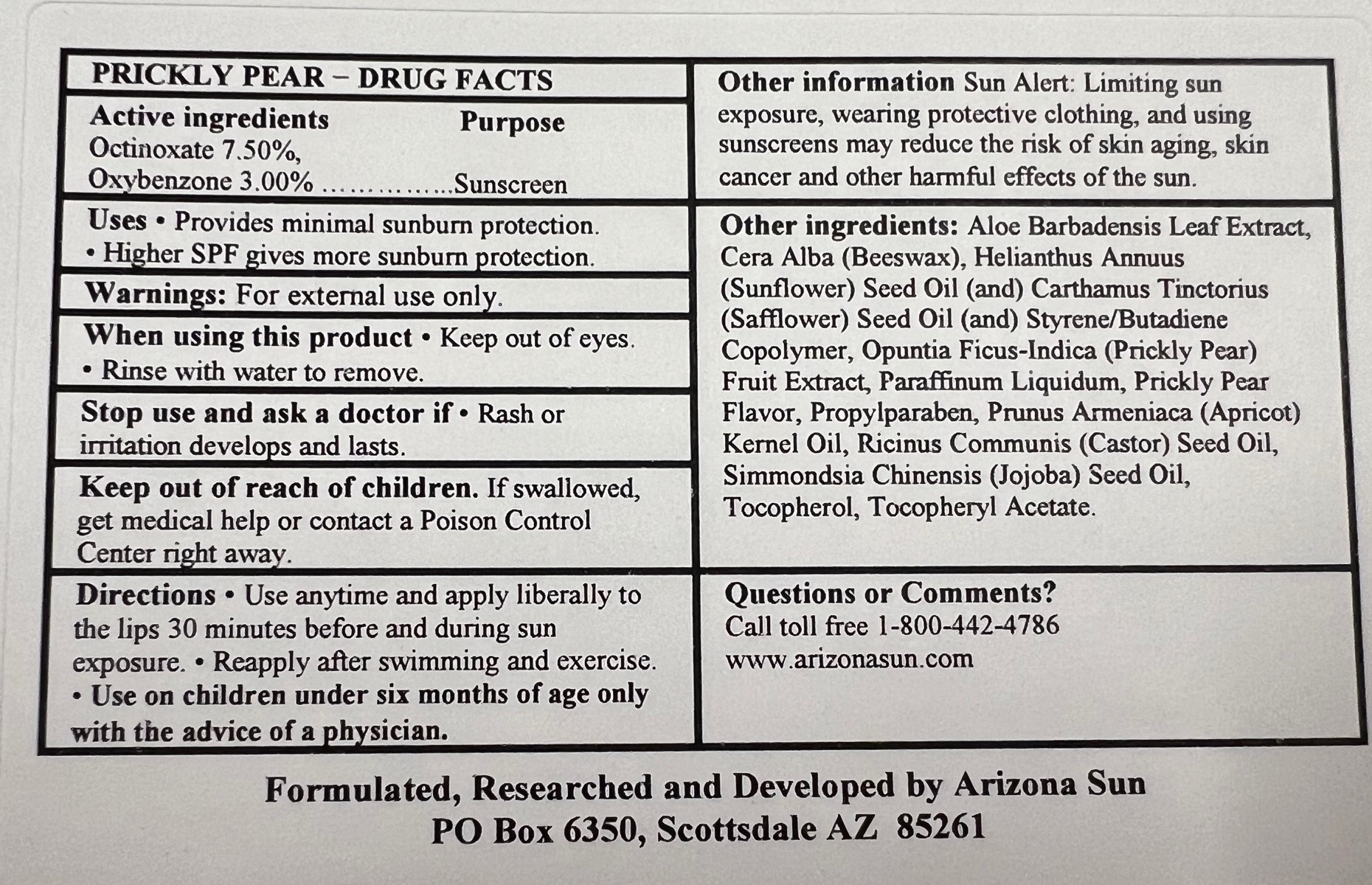
- Drug Facts
- Active Ingredients
- Purpose
- Uses
- Warnings
- Directions
- Other Information
-
Inactive Ingredients
Aloe Barbadensis Leaf (Aloe Vera) Extract, Cera Alba (Beeswax), Helianthus Annuus (Sunflower) Seed Oil, Carthamus Tinctorius (Safflower) Seed Oil, Styrene/Butadiene Copolymer, Opuntia Ficus-Indica (Prickly Pear) Fruit Extract, Paraffinum Liquidum, Prickly Pear Flavor, Propylparaben, Prunus Armeniaca (Apricot) Kernel Oil, Ricinus Communis (Castor) Seed Oil, Simmondsia Chinensis (Jojoba) Seed Oil, Tocopherol, Tocopheryl Acetate
- Questions or Comments?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ARIZONA SUN PRICKLY PEAR LIP BALM SPF 15
sunscreen lip balm lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:61973-400 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 75 mg in 1 g OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 30 mg in 1 g Inactive Ingredients Ingredient Name Strength PRICKLY PEAR FRUIT (UNII: 18V8PAQ629) TOCOPHEROL (UNII: R0ZB2556P8) OPUNTIA FICUS-INDICA FRUIT JUICE (UNII: 5ZC110ZY2H) PROPYLPARABEN (UNII: Z8IX2SC1OH) SAFFLOWER OIL (UNII: 65UEH262IS) ALOE VERA LEAF (UNII: ZY81Z83H0X) MINERAL OIL (UNII: T5L8T28FGP) CASTOR OIL (UNII: D5340Y2I9G) SUNFLOWER SEED (UNII: R9N3379M4Z) SIMMONDSIA CHINENSIS SEED (UNII: D24K2Q1F6H) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) WHITE WAX (UNII: 7G1J5DA97F) APRICOT KERNEL OIL (UNII: 54JB35T06A) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61973-400-10 153 g in 1 CARTON; Type 1: Convenience Kit of Co-Package 02/21/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 02/21/2024 Labeler - Arizona Sun Products (107220212)