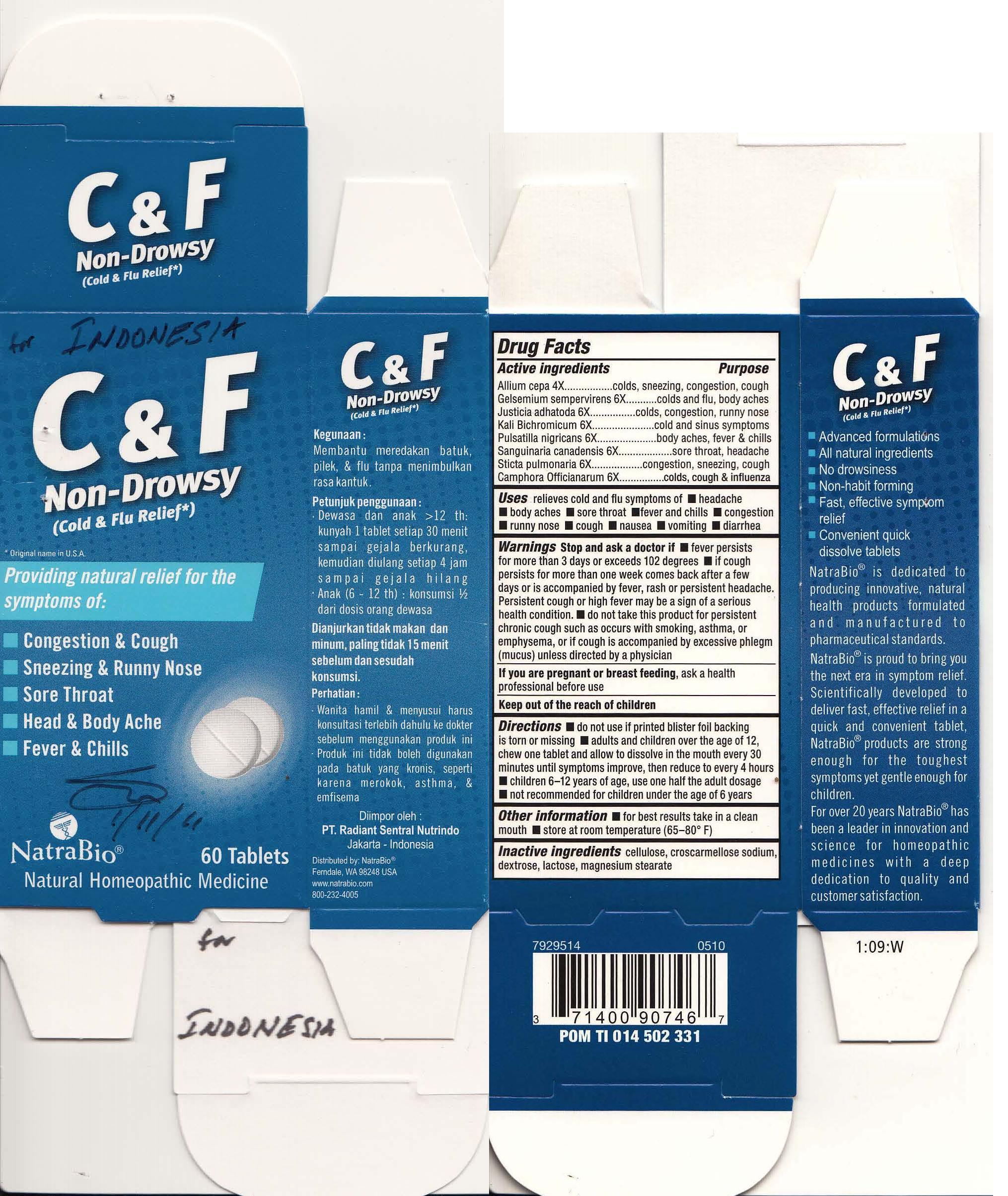
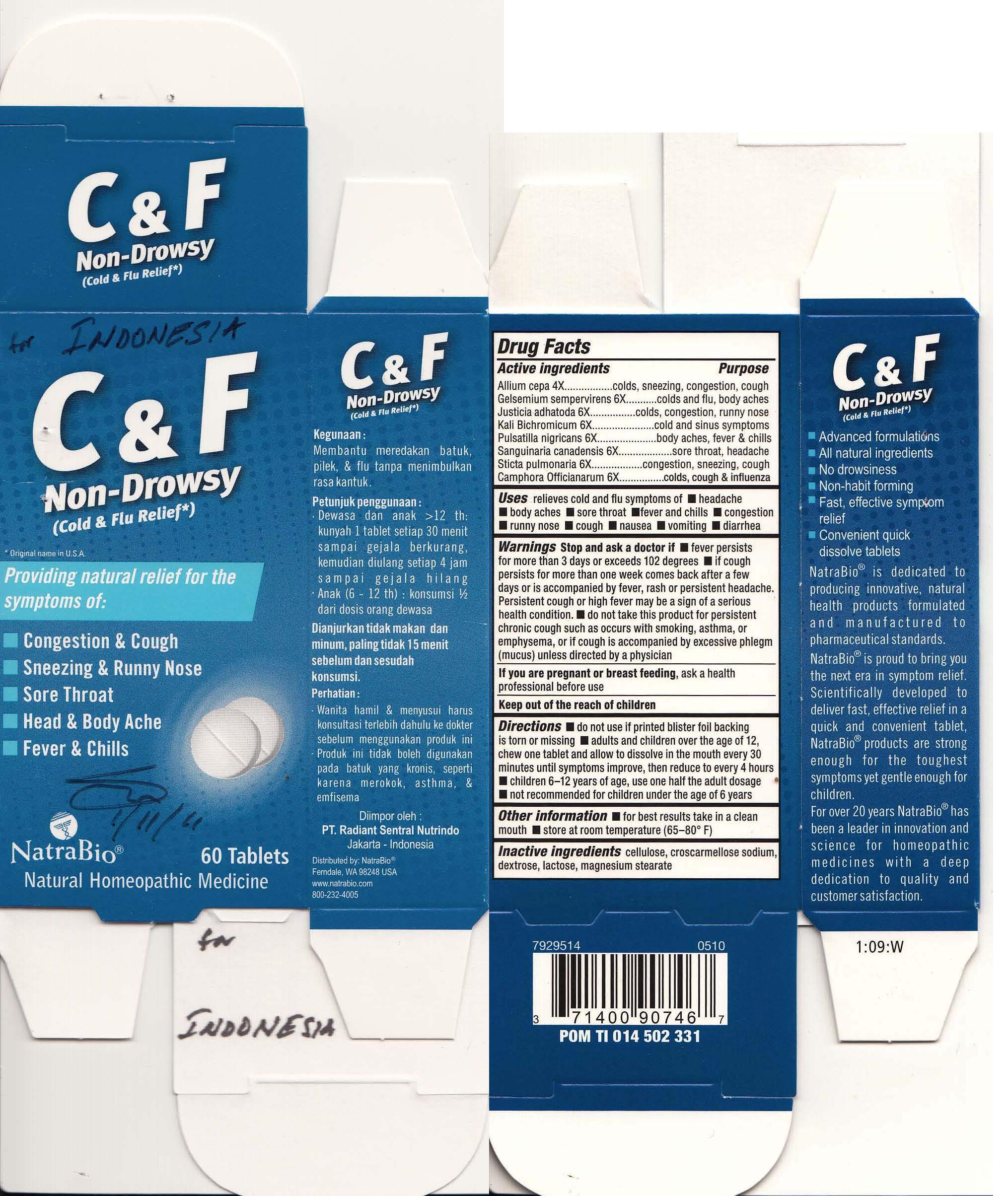
Label: COLD AND FLU RELIEF- aconitum napellus, allium cepa, gelsemium supervirens, justica adhatoda, kali bichromicum, pulsatill nigricans, sanguinaria canadensis, sticta pulmonaria, camphora offcianarum tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 65808-024-01 - Packager: GMP Laboratories of America, Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated June 26, 2015
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Drug Facts
Active ingredient Purpose Aconitum napellus influenza, colds, fever, congestion Allium cepa colds, sneezing, congestion, cough Gelsemium sempervirens colds and flu, body aches Justicia adhatoda colds, congestion, runny nose Kali Bichromicum cold and sinus symptoms Pulsatilla nigricans body aches, fever & chills Sanguinaria canadensis sore throat, headache Sticta pulmonaria congestions, sneezing, cough Camphora Offcianarum colds, cough & influenza - Uses
-
Warnings
Stop and ask a doctor if
- fever persists for more than 3 days or exceeds 102 degrees
- if cough persists for more than one week, comes back after a few days, or is accompanied by fever, rash or persistent headache. Persistent cough or high fever may be a sign of a serious health condition.
- do not take this product for persistent chronic cough such as occurs with smoking, asthma, or emphysema, or if cough is accompanied by excessive phlegm (mucus) unless directed by a physician
-
Directions
- do not use if printed blister foil backing is torn or missing
- adults and children over the age of 12, chew one tablet and allow to dissolve in the mouth every 30 minutes until symptoms inprove, then reduce to every 4 hours
- children 6-12 use one half the adult dosage
- not recommended for children under the age of 6
- Other information
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
COLD AND FLU RELIEF
aconitum napellus, allium cepa, gelsemium supervirens, justica adhatoda, kali bichromicum, pulsatill nigricans, sanguinaria canadensis, sticta pulmonaria, camphora offcianarum tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:65808-024 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACONITUM NAPELLUS (UNII: U0NQ8555JD) (ACONITUM NAPELLUS - UNII:U0NQ8555JD) ACONITUM NAPELLUS 4 [hp_X] ONION (UNII: 492225Q21H) (ONION - UNII:492225Q21H) ONION 4 [hp_X] GELSEMIUM SEMPERVIRENS ROOT (UNII: 639KR60Q1Q) (GELSEMIUM SEMPERVIRENS ROOT - UNII:639KR60Q1Q) GELSEMIUM SEMPERVIRENS ROOT 4 [hp_X] JUSTICIA ADHATODA LEAF (UNII: HH159XOV81) (JUSTICIA ADHATODA LEAF - UNII:HH159XOV81) JUSTICIA ADHATODA LEAF 6 [hp_X] POTASSIUM CHROMATE (UNII: 5P0R38CN2X) (POTASSIUM CATION - UNII:295O53K152) POTASSIUM CHROMATE 6 [hp_X] PULSATILLA VULGARIS (UNII: I76KB35JEV) (PULSATILLA VULGARIS - UNII:I76KB35JEV) PULSATILLA VULGARIS 6 [hp_X] SANGUINARIA CANADENSIS ROOT (UNII: N9288CD508) (SANGUINARIA CANADENSIS ROOT - UNII:N9288CD508) SANGUINARIA CANADENSIS ROOT 6 [hp_X] LOBARIA PULMONARIA (UNII: D1YM0P5Z2T) (LOBARIA PULMONARIA - UNII:D1YM0P5Z2T) LOBARIA PULMONARIA 6 [hp_X] CAMPHOR (NATURAL) (UNII: N20HL7Q941) (CAMPHOR (NATURAL) - UNII:N20HL7Q941) CAMPHOR (NATURAL) 6 [hp_X] Inactive Ingredients Ingredient Name Strength POWDERED CELLULOSE (UNII: SMD1X3XO9M) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) DEXTROSE (UNII: IY9XDZ35W2) LACTOSE (UNII: J2B2A4N98G) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color WHITE (White) Score 2 pieces Shape ROUND Size 10mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65808-024-01 6 in 1 CARTON 1 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 10/21/2009 Labeler - GMP Laboratories of America, Inc (876754375) Establishment Name Address ID/FEI Business Operations GMP Laboratories of America, Inc 876754375 MANUFACTURE(65808-024)