Label: INGREZZA- valbenazine capsule
INGREZZA- valbenazine kit
INGREZZA SPRINKLE- valbenazine capsule
-
NDC Code(s):
70370-1060-1,
70370-1080-1,
70370-2040-1,
70370-2046-1, view more70370-2048-6, 70370-4040-1, 70370-4060-1, 70370-4080-1
- Packager: Neurocrine Biosciences, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated April 30, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use INGREZZA or INGREZZA SPRINKLE safely and effectively. See full prescribing information for INGREZZA.
INGREZZA® (valbenazine) capsules, for oral use
INGREZZA® SPRINKLE (valbenazine) capsules, for oral use
Initial U.S. Approval: 2017
WARNING: DEPRESSION AND SUICIDAL IDEATION AND BEHAVIOR IN PATIENTS WITH HUNTINGTON’S DISEASE
See full prescribing information for complete boxed warning.
• Increases the risk of depression and suicidal thoughts and behavior in patients with Huntington’s disease (5.1)
• Balance risks of depression, and suicidal ideation and behavior with the clinical need for treatment of chorea when considering the use of INGREZZA or INGREZZA SPRINKLE (5.1)
• Monitor patients for the emergence or worsening of depression, suicidal ideation, or unusual changes in behavior (5.1)
• Inform patients, caregivers, and families of the risk of depression and suicidal ideation and behavior and instruct them to report behaviors of concern promptly to the treating physician (5.1)
• Exercise caution when treating patients with a history of depression or prior suicide attempts or ideation (5.1)
RECENT MAJOR CHANGES
INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
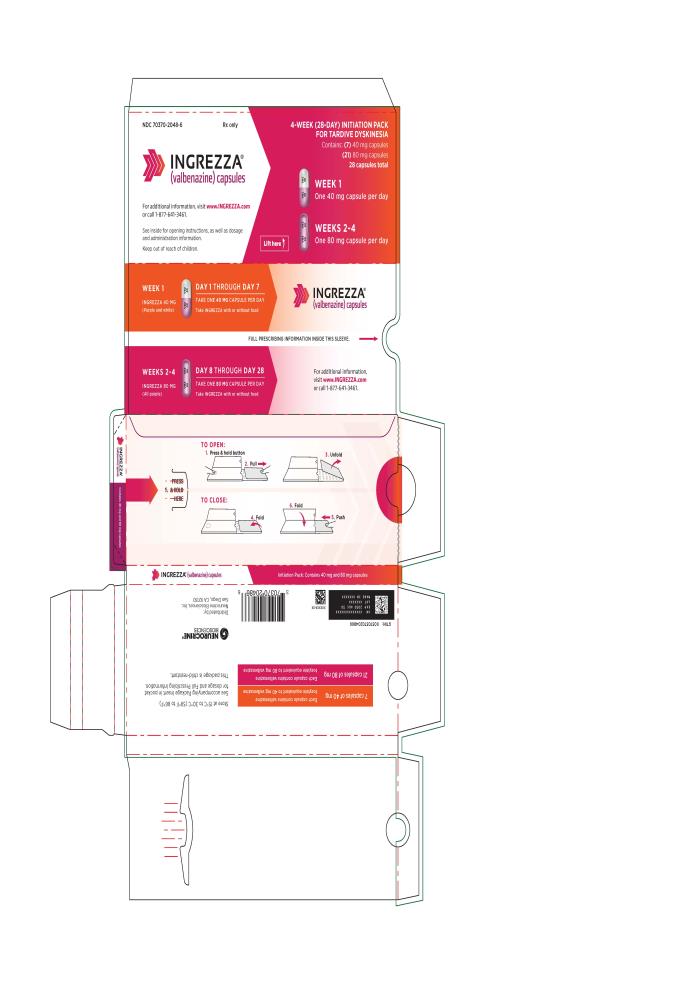
- Tardive dyskinesia: The initial dosage is 40 mg once daily. After one week, increase the dose to the recommended dosage of 80 mg once daily. (2.1)
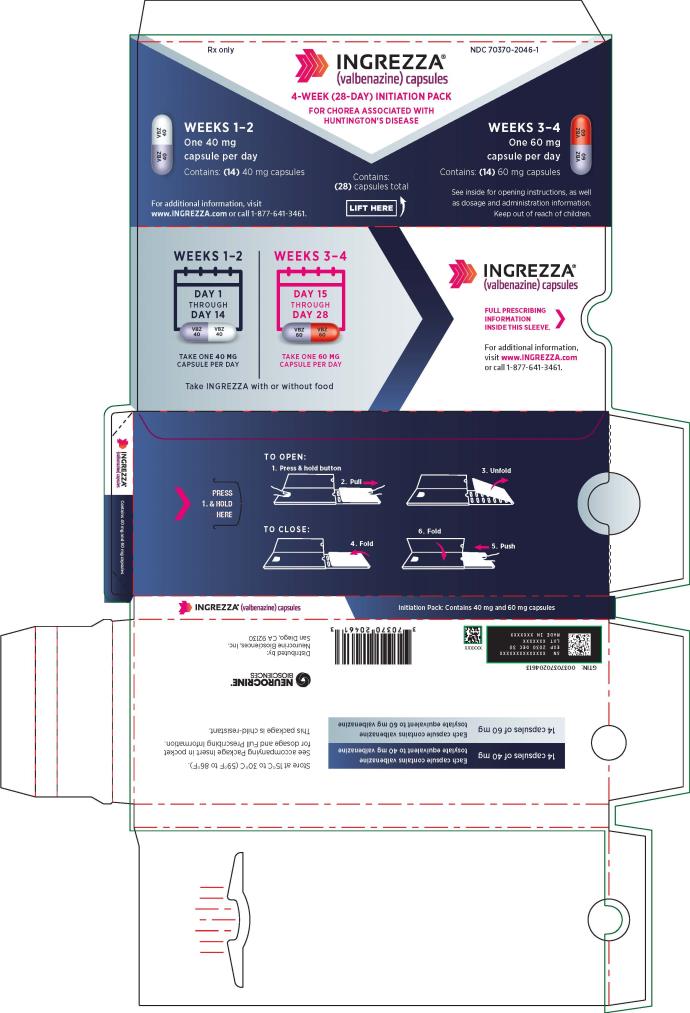
- Chorea associated with Huntington’s disease: The initial dosage is 40 mg once daily. Increase the dose in 20 mg increments every two weeks to the recommended dosage of 80 mg once daily. (2.1)
- 40 mg or 60 mg once daily may be considered depending on response and tolerability. (2.1)
- Can be taken with or without food. (2.2)
- INGREZZA SPRINKLE may be opened and sprinkled over soft food (do not use milk or drinking water). INGREZZA SPRINKLE may be swallowed whole with water. Do not crush or chew. (2.2)
- The recommended dosage for patients with moderate or severe hepatic impairment is 40 mg once daily. (2.3)
- The recommended dosage for known CYP2D6 poor metabolizers is 40 mg once daily. (2.4)
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
Known hypersensitivity to valbenazine or any components of INGREZZA or INGREZZA SPRINKLE. (4)
WARNINGS AND PRECAUTIONS
- Depression and suicidal ideation and behavior in patients with Huntington’s disease. (5.1)
- Hypersensitivity, including angioedema may occur. Discontinue if this occurs. (5.2)
- Somnolence/sedation: May impair patient’s ability to drive or operate hazardous machinery. (5.3)
- QT Prolongation: May cause an increase in QT interval. Avoid use in patients with congenital long QT syndrome or with arrhythmias associated with a prolonged QT interval. (5.4)
- Neuroleptic Malignant Syndrome (NMS): Discontinue if this occurs. (5.5)
- Parkinsonism: Cases of parkinson-like symptoms, some of which were severe, have been reported in the postmarketing period. Reduce the dose or discontinue INGREZZA or INGREZZA SPRINKLE treatment in patients who develop clinically significant parkinson-like signs or symptoms. (5.6)
ADVERSE REACTIONS
Most common adverse reaction (≥5% and twice the rate of placebo):
- Tardive dyskinesia: somnolence. (6.1)
- Chorea associated with Huntington’s disease: somnolence/lethargy/sedation, urticaria, rash, insomnia. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Neurocrine Biosciences, Inc. at 877-641-3461 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.DRUG INTERACTIONS
Dose adjustments due to drug interactions (2.4, 7.1):
Factors Dose Adjustments for INGREZZA and INGREZZA SPRINKLE Use of MAOIs with INGREZZA or INGREZZA SPRINKLE Avoid concomitant use with MAOIs. Use of strong CYP3A4 inducers with INGREZZA or INGREZZA SPRINKLE Concomitant use is not recommended. Use of strong CYP3A4 inhibitors with INGREZZA or INGREZZA SPRINKLE Recommended dosage is 40 mg once daily.
Use of strong CYP2D6 inhibitors with INGREZZA or INGREZZA SPRINKLE Recommended dosage is 40 mg once daily. USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 4/2024
- Tardive dyskinesia: The initial dosage is 40 mg once daily. After one week, increase the dose to the recommended dosage of 80 mg once daily. (2.1)
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: DEPRESSION AND SUICIDAL IDEATION AND BEHAVIOR IN PATIENTS WITH HUNTINGTON’S DISEASE
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 Administrative Information
2.3 Dosage Recommendations for Patients with Hepatic Impairment
2.4 Dosage Recommendations for Known CYP2D6 Poor Metabolizers
2.5 Dosage Recommendations for Concomitant Use with Strong CYP3A4 Inducers and Strong CYP3A4 or CYP2D6 Inhibitors
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Depression and Suicidal Ideation and Behavior in Patients with Huntington’s Disease
5.2 Hypersensitivity Reactions
5.3 Somnolence and Sedation
5.4 QT Prolongation
5.5 Neuroleptic Malignant Syndrome (NMS)
5.6 Parkinsonism
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Drugs Having Clinically Important Interactions with INGREZZA and INGREZZA SPRINKLE
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 CYP2D6 Poor Metabolizers
8.7 Hepatic Impairment
8.8 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.5 Pharmacogenomics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Tardive Dyskinesia
14.2 Chorea Associated with Huntington’s Disease
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: DEPRESSION AND SUICIDAL IDEATION AND BEHAVIOR IN PATIENTS WITH HUNTINGTON’S DISEASE
VMAT2 inhibitors, including INGREZZA and INGREZZA SPRINKLE, can increase the risk of depression and suicidal thoughts and behavior in patients with Huntington’s disease. Anyone considering the use of INGREZZA or INGREZZA SPRINKLE must balance the risks of depression and suicidal ideation and behavior with the clinical need for treatment of chorea. Closely monitor patients for the emergence or worsening of depression, suicidal ideation, or unusual changes in behavior. Inform patients, their caregivers, and families of the risk of depression and suicidal ideation and behavior and instruct them to report behaviors of concern promptly to the treating physician.
Particular caution should be exercised in treating patients with a history of depression or prior suicide attempts or ideation, which are increased in frequency in patients with Huntington’s disease [see Warnings and Precautions (5.1)].
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
Tardive Dyskinesia
The initial dosage for INGREZZA and INGREZZA SPRINKLE is 40 mg once daily. After one week, increase the dose to the recommended dosage of 80 mg once daily. A dosage of 40 mg or 60 mg once daily may be considered depending on response and tolerability.
Chorea Associated with Huntington’s Disease
The initial dosage for INGREZZA and INGREZZA SPRINKLE is 40 mg once daily. Increase the dose in 20 mg increments every two weeks to the recommended dosage of 80 mg once daily. A dosage of 40 mg or 60 mg once daily may be considered depending on response and tolerability.
2.2 Administrative Information
Administer INGREZZA and INGREZZA SPRINKLE orally with or without food [see Clinical Pharmacology (12.3)].
Administration Information for INGREZZA SPRINKLE
• Open INGREZZA SPRINKLE and sprinkle the entire contents of the capsule over a bowl containing a small amount (1 tablespoonful) of soft food such as applesauce, yogurt, or pudding. Do not sprinkle the contents of the capsule into milk or drinking water. Stir the contents of the capsule into the soft food with the tablespoon and swallow the drug/food mixture immediately. If necessary, the mixture can be stored for up to 2 hours at room temperature. Discard of any unused portion after 2 hours. Following administration of the drug/food mixture, drink a glass (e.g., 240 mL) of water.
Do not administer INGREZZA SPRINKLE via nasogastric, gastrostomy, or other enteral tubes because it may cause obstruction of enteral tubes.
• INGREZZA SPRINKLE may be swallowed whole with water. Do not crush or chew INGREZZA SPRINKLE.
2.3 Dosage Recommendations for Patients with Hepatic Impairment
The recommended dosage for patients with moderate or severe hepatic impairment (Child-Pugh score 7 to 15) is INGREZZA or INGREZZA SPRINKLE 40 mg once daily [see Use in Specific Populations (8.7), Clinical Pharmacology (12.3)].
2.4 Dosage Recommendations for Known CYP2D6 Poor Metabolizers
The recommended dosage for known CYP2D6 poor metabolizers is INGREZZA or INGREZZA SPRINKLE 40 mg once daily [see Use in Specific Populations (8.6), Clinical Pharmacology (12.3, 12.5)].
2.5 Dosage Recommendations for Concomitant Use with Strong CYP3A4 Inducers and Strong CYP3A4 or CYP2D6 Inhibitors
Coadministration with Strong CYP3A4 Inducers
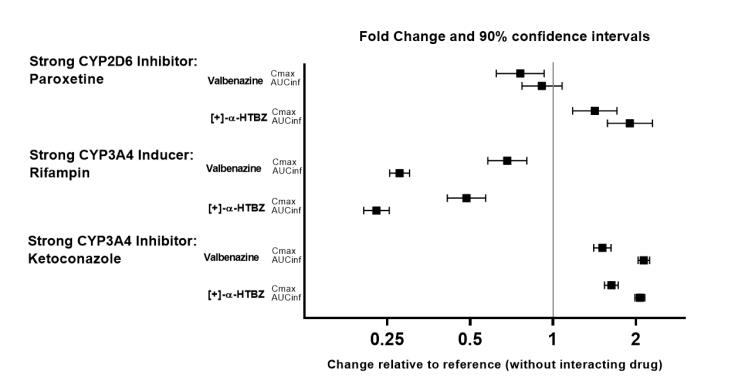
Concomitant use of strong CYP3A4 inducers with INGREZZA or INGREZZA SPRINKLE is not recommended [see Drug Interactions (7.1)].
Coadministration with Strong CYP3A4 Inhibitors
The recommended dosage for patients receiving strong CYP3A4 inhibitors is INGREZZA or INGREZZA SPRINKLE 40 mg once daily [see Drug Interactions (7.1)].
Coadministration with Strong CYP2D6 Inhibitors
The recommended dosage for patients receiving strong CYP2D6 inhibitors is INGREZZA or INGREZZA SPRINKLE 40 mg once daily [see Drug Interactions (7.1)].
-
3 DOSAGE FORMS AND STRENGTHS
INGREZZA
- 40 mg capsule: white opaque body and purple cap, printed with ‘VBZ’ and ‘40’ in black ink. Each capsule contains 40 mg valbenazine.
- 60 mg capsule: dark red opaque body and purple cap, printed with ‘VBZ’ and ‘60’ in black ink. Each capsule contains 60 mg valbenazine.
- 80 mg capsule: purple opaque body and cap, printed with ‘VBZ’ and ‘80’ in black ink. Each capsule contains 80 mg valbenazine.
INGREZZA SPRINKLE
- 40 mg capsule: pearl white opaque cap and body, printed with a band, directional arrows, and “VBZ 40” in black ink on both the cap and body. Each capsule contains 40 mg valbenazine.
- 60 mg capsule: pearl white opaque cap and body, printed with a band, directional arrows, and “VBZ 60” in dark red ink on both the cap and body. Each capsule contains 60 mg valbenazine.
- 80 mg capsule: pearl white opaque cap and body, printed with a band, directional arrows, and “VBZ 80” in purple ink on both the cap and body. Each capsule contains 80 mg valbenazine.
- 40 mg capsule: white opaque body and purple cap, printed with ‘VBZ’ and ‘40’ in black ink. Each capsule contains 40 mg valbenazine.
-
4 CONTRAINDICATIONS
INGREZZA and INGREZZA SPRINKLE are contraindicated in patients with a history of hypersensitivity to valbenazine or any components of INGREZZA or INGREZZA SPRINKLE. Rash, urticaria, and reactions consistent with angioedema (e.g., swelling of the face, lips, and mouth) have been reported with use of INGREZZA [see Warnings and Precautions (5.2) and Adverse Reactions (6.2)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Depression and Suicidal Ideation and Behavior in Patients with Huntington’s Disease
Patients with Huntington’s disease are at increased risk for depression, and suicidal ideation or behaviors. VMAT2 inhibitors, including INGREZZA and INGREZZA SPRINKLE, can increase the risk for suicidal ideation and behaviors in patients with Huntington’s disease.
In a 14-week, double-blind, placebo-controlled trial [see Clinical Studies (14.2)], depression or depressed mood was reported in 4.7% of patients taking INGREZZA compared to 1.6% of patients who received placebo, and no patients taking INGREZZA reported suicidal ideation or behavior compared to 1 patient (1.6%) who received placebo. Patients with significant risk for suicidal behavior or with unstable psychiatric symptoms were excluded from this trial. Suicidal ideation (9 subjects; 7.2%) and suicide attempts (3 subjects; 2.4%) were reported in the longer open-label extension trial (N=125).
When considering the use of INGREZZA or INGREZZA SPRINKLE, the risk of suicidal ideation and behaviors must be balanced against the need for treatment of chorea. All patients treated with INGREZZA and INGREZZA SPRINKLE should be observed for new or worsening depression, suicidal ideation or behaviors. If any of these reactions occur and do not resolve, consider discontinuing treatment with INGREZZA or INGREZZA SPRINKLE.
5.2 Hypersensitivity Reactions
Hypersensitivity reactions, including cases of angioedema involving the larynx, glottis, lips, and eyelids, have been reported in the postmarketing setting in patients after taking the first or subsequent doses of INGREZZA [see Adverse Reactions (6.2)]. A case of angioedema involving the lips and face, with rash and shortness of breath was reported in a patient with Huntington’s disease taking INGREZZA during a clinical study. Urticaria and rash were also reported during a clinical study in patients with Huntington’s disease. Angioedema associated with laryngeal edema can be fatal. If any of these reactions occur, discontinue INGREZZA or INGREZZA SPRINKLE.
5.3 Somnolence and Sedation
INGREZZA and INGREZZA SPRINKLE can cause somnolence and sedation, which was the most common adverse reaction in placebo-controlled trials with INGREZZA [see Adverse Reactions (6.1)]. Patients should not perform activities requiring mental alertness such as operating a motor vehicle or operating hazardous machinery until they know how they will be affected by INGREZZA or INGREZZA SPRINKLE.
5.4 QT Prolongation
INGREZZA and INGREZZA SPRINKLE may prolong the QT interval, although the degree of QT prolongation is not clinically significant at concentrations expected with recommended dosing. In patients taking a strong CYP2D6 or CYP3A4 inhibitor, or who are CYP2D6 poor metabolizers, INGREZZA and INGREZZA SPRINKLE concentrations may be higher and QT prolongation clinically significant [see Clinical Pharmacology (12.2)]. For patients who are CYP2D6 poor metabolizers or are taking a strong CYP2D6 inhibitor, dose reduction may be necessary. For patients taking a strong CYP3A4 inhibitor, reduce the dose of INGREZZA or INGREZZA SPRINKLE to 40 mg once daily [see Dosage and Administration (2.4, 2.5)]. INGREZZA and INGREZZA SPRINKLE should be avoided in patients with congenital long QT syndrome or with arrhythmias associated with a prolonged QT interval. For patients at increased risk of a prolonged QT interval, assess the QT interval before increasing the dosage.
5.5 Neuroleptic Malignant Syndrome (NMS)
A potentially fatal symptom complex referred to as Neuroleptic Malignant Syndrome (NMS) has been reported in association with drugs that reduce dopaminergic transmission. In the postmarketing setting, NMS has been reported in patients taking VMAT2 inhibitors, including INGREZZA. Clinicians should be alerted to the signs and symptoms associated with NMS. Clinical manifestations of NMS are hyperpyrexia, muscle rigidity, altered mental status, and evidence of autonomic instability (irregular pulse or blood pressure, tachycardia, diaphoresis, and cardiac dysrhythmia). Additional signs may include elevated creatine phosphokinase, myoglobinuria, rhabdomyolysis, and acute renal failure. The diagnosis of NMS can be complicated; other serious medical illness (e.g., pneumonia, systemic infection) and untreated or inadequately treated extrapyramidal disorders can present with similar signs and symptoms. Other important considerations in the differential diagnosis include central anticholinergic toxicity, heat stroke, drug fever, and primary central nervous system pathology.
The management of NMS should include (1) immediate discontinuation of INGREZZA or INGREZZA SPRINKLE; (2) intensive symptomatic treatment and medical monitoring; and (3) treatment of any concomitant serious medical problems for which specific treatments are available. There is no general agreement about specific pharmacological treatment regimens for NMS.
Recurrence of NMS has been reported with resumption of drug therapy. If treatment with INGREZZA or INGREZZA SPRINKLE is needed after recovery from NMS, patients should be monitored for signs of recurrence.
5.6 Parkinsonism
INGREZZA and INGREZZA SPRINKLE may cause parkinsonism. Parkinsonism has also been observed with other VMAT2 inhibitors. In the 3 placebo-controlled clinical studies in patients with tardive dyskinesia, the incidence of parkinson-like adverse events was 3% of patients treated with INGREZZA and <1% of placebo-treated patients.
In a placebo-controlled clinical study in patients with chorea associated with Huntington’s disease, the incidence of parkinson-like adverse events was 4.7% in patients treated with INGREZZA and 0% in placebo-treated patients. Because rigidity can develop as part of the underlying disease process in Huntington’s disease, it may be difficult to distinguish between potential drug-induced parkinsonism and progression of underlying Huntington’s disease. Drug-induced parkinsonism has the potential to cause more functional disability than untreated chorea for some patients with Huntington’s disease.
Postmarketing safety reports have described parkinson-like symptoms in patients taking INGREZZA for tardive dyskinesia, some of which were severe and required hospitalization. In most cases, severe parkinsonism occurred within the first two weeks after starting or increasing the dose of INGREZZA. Associated symptoms have included falls, gait disturbances, tremor, drooling and hypokinesia. In cases in which follow-up clinical information was available, parkinson-like symptoms were reported to resolve following discontinuation of INGREZZA therapy.
Reduce the dose or discontinue INGREZZA or INGREZZA SPRINKLE treatment in patients who develop clinically significant parkinson-like signs or symptoms.
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are discussed in more detail in other sections of the labeling:
- Depression and Suicidal Ideation and Behavior in Patients with Huntington’s Disease [see Boxed Warning and Warnings and Precautions (5.1)]
- Hypersensitivity Reactions [see Contraindications (4) and Warnings and Precautions (5.2)]
- Somnolence and Sedation [see Warnings and Precautions (5.3)]
- QT Prolongation [see Warnings and Precautions (5.4)]
- Neuroleptic Malignant Syndrome (NMS) [see Warnings and Precautions (5.5)]
- Parkinsonism [see Warnings and Precautions (5.6)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of INGREZZA SPRINKLE has been established from adequate and well-controlled studies of INGREZZA [see Clinical Studies (14)]. Below is a display of the adverse reactions of INGREZZA in these adequate and well-controlled studies.
Tardive Dyskinesia
Variable and Fixed Dose Placebo-Controlled Trial Experience
The safety of INGREZZA was evaluated in 3 placebo-controlled studies, each 6 weeks in duration (fixed dose, dose escalation, dose reduction), including 445 patients. Patients were 26 to 84 years of age with moderate to severe tardive dyskinesia and had concurrent diagnoses of mood disorder (27%) or schizophrenia/ schizoaffective disorder (72%). The mean age was 56 years. Patients were 57% Caucasian, 39% African-American, and 4% other. With respect to ethnicity, 28% were Hispanic or Latino. All subjects continued previous stable regimens of antipsychotics; 85% and 27% of subjects, respectively, were taking atypical and typical antipsychotic medications at study entry.
Adverse Reactions Leading to Discontinuation of Treatment
A total of 3% of INGREZZA-treated patients and 2% of placebo-treated patients discontinued because of adverse reactions.
Common Adverse Reactions
Adverse reactions that occurred in the 3 placebo-controlled studies at an incidence of ≥2% and greater than placebo are presented in Table 1.
Table 1: Adverse Reactions in 3 Placebo-Controlled Studies of 6-week Treatment Duration Reported at ≥2% and >Placebo – Tardive Dyskinesia Adverse Reaction1 INGREZZA
(n=262)
%Placebo
(n=183)
%General Disorders Somnolence
(somnolence, fatigue, sedation)10.9 4.2 Nervous System Disorders Anticholinergic effects 5.4 4.9 (dry mouth, constipation, disturbance in attention, vision blurred, urinary retention) Balance disorders/fall
(fall, gait disturbance, dizziness, balance disorder)4.1 2.2 Headache 3.4 2.7 Akathisia
(akathisia, restlessness)2.7 0.5 Gastrointestinal Disorders Vomiting 2.6 0.6 Nausea 2.3 2.1 Musculoskeletal Disorders Arthralgia 2.3 0.5 1 Within each adverse reaction category, the observed adverse reactions are listed in order of decreasing frequency.
Other Adverse Reactions Observed During the Premarketing Evaluation of INGREZZA
Other adverse reactions of ≥1% incidence and greater than placebo are shown below. The following list does not include adverse reactions: 1) already listed in previous tables or elsewhere in the labeling, 2) for which a drug cause was remote, 3) which were so general as to be uninformative, 4) which were not considered to have clinically significant implications, or 5) which occurred at a rate equal to or less than placebo.
Endocrine Disorders: blood glucose increased
General Disorders: weight increased
Infectious Disorders: respiratory infections
Neurologic Disorders: drooling, dyskinesia, extrapyramidal symptoms (non-akathisia)
Psychiatric Disorders: anxiety, insomnia
During the tardive dyskinesia controlled trials, there was a dose-related increase in prolactin. Additionally, in these trials there was a dose-related increase in alkaline phosphatase and bilirubin, suggesting a potential risk for cholestasis.
Chorea Associated with Huntington’s Disease
The safety of INGREZZA was evaluated in a 14-week placebo-controlled study including 127 patients with chorea associated with Huntington’s disease. Patients were 25 to 75 years of age. The mean age was 54 years. Patients were 96% Caucasian, 1% African-American, 1% Asian, and 2% Other. With respect to ethnicity, 6% were Hispanic or Latino.
Adverse Reactions Leading to Discontinuation of Treatment
A total of 8% of INGREZZA-treated patients and 6% of placebo-treated patients discontinued because of adverse reactions.
Common Adverse Reactions
Adverse reactions that occurred in the placebo-controlled study at an incidence of ≥4% and greater than placebo are presented in Table 2.
Table 2: Adverse Reactions in the Placebo-Controlled Study of 12-week Treatment Duration Reported at ≥4% and >Placebo – Chorea Associated with Huntington’s Disease Adverse Reaction INGREZZA
(n=64)
%Placebo
(n=63)
%Nervous System Disorders Somnolence, lethargy, sedation 18.8 3.2 Akathisia 6.3 4.8 General Disorders and Administration Site Conditions Fatigue 14.1 9.5 Skin and Subcutaneous Tissue Disorders Urticaria 9.4 0 Rash 7.8 0 Gastrointestinal Disorders Diarrhea 4.7 1.6 Nausea 4.7 0 Psychiatric Disorders Insomnia, middle insomnia 6.3 1.6 Depression, depressed mood 4.7 1.6 Musculoskeletal Disorders Back pain 4.7 0 6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of INGREZZA that are not included in other sections of labeling. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Immune System Disorders: hypersensitivity reactions (including allergic dermatitis and pruritis)
- Depression and Suicidal Ideation and Behavior in Patients with Huntington’s Disease [see Boxed Warning and Warnings and Precautions (5.1)]
-
7 DRUG INTERACTIONS
7.1 Drugs Having Clinically Important Interactions with INGREZZA and INGREZZA SPRINKLE
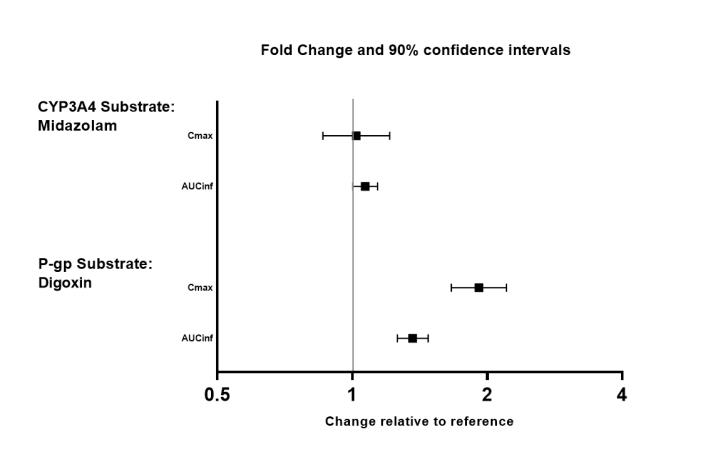
Table 3: Clinically Significant Drug Interactions with INGREZZA and INGREZZA SPRINKLE Monoamine Oxidase Inhibitors (MAOIs) Clinical Implication: Concomitant use of INGREZZA or INGREZZA SPRINKLE with MAOIs may increase the concentration of monoamine neurotransmitters in synapses, potentially leading to increased risk of adverse reactions such as serotonin syndrome, or attenuated treatment effect of INGREZZA or INGREZZA SPRINKLE. Prevention or Management: Avoid concomitant use of INGREZZA or INGREZZA SPRINKLE with MAOIs, or within 14 days of discontinuing therapy with an MAOI. Strong CYP3A4 Inhibitors Clinical Implication: Concomitant use of INGREZZA or INGREZZA SPRINKLE with strong CYP3A4 inhibitors increased the exposure (Cmax and AUC) to valbenazine and its active metabolite compared with the use of INGREZZA or INGREZZA SPRINKLE alone [see Clinical Pharmacology (12.3)]. Increased exposure of valbenazine and its active metabolite may increase the risk of exposure-related adverse reactions [see Warnings and Precautions (5.4)]. Prevention or Management: Reduce INGREZZA or INGREZZA SPRINKLE dose when INGREZZA or INGREZZA SPRINKLE is coadministered with a strong CYP3A4 inhibitor [see Dosage and Administration (2.5)]. Strong CYP2D6 Inhibitors Clinical Implication: Concomitant use of INGREZZA or INGREZZA SPRINKLE with strong CYP2D6 inhibitors increased the exposure (Cmax and AUC) to valbenazine’s active metabolite compared with the use of INGREZZA or INGREZZA SPRINKLE alone [see Clinical Pharmacology (12.3, 12.5)]. Increased exposure of active metabolite may increase the risk of exposure-related adverse reactions [see Warnings and Precautions (5.4)]. Prevention or Management: Reduce INGREZZA or INGREZZA SPRINKLE dose when INGREZZA or INGREZZA SPRINKLE is coadministered with a strong CYP2D6 inhibitor [see Dosage and Administration (2.5)]. Strong CYP3A4 Inducers Clinical Implication: Concomitant use of INGREZZA or INGREZZA SPRINKLE with a strong CYP3A4 inducer decreased the exposure of valbenazine and its active metabolite compared to the use of INGREZZA or INGREZZA SPRINKLE alone. Reduced exposure of valbenazine and its active metabolite may reduce efficacy [see Clinical Pharmacology (12.3)]. Prevention or Management: Concomitant use of strong CYP3A4 inducers with INGREZZA or INGREZZA SPRINKLE is not recommended [see Dosage and Administration (2.5)]. Digoxin Clinical Implication: Concomitant use of INGREZZA or INGREZZA SPRINKLE with digoxin increased digoxin levels because of inhibition of intestinal P-glycoprotein (P-gp) [see Clinical Pharmacology (12.3)]. Prevention or Management:
Digoxin concentrations should be monitored when co-administering INGREZZA or INGREZZA SPRINKLE with digoxin. Increased digoxin exposure may increase the risk of exposure-related adverse reactions. Dosage adjustment of digoxin may be necessary. -
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
The limited available data on INGREZZA or INGREZZA SPRINKLE use in pregnant women are insufficient to inform a drug-associated risk. In animal reproductive studies, no malformations were observed when valbenazine was administered orally to rats and rabbits during the period of organogenesis at doses up to 1.8 or 24 times, respectively, the maximum recommended human dose (MRHD) of 80 mg/day based on mg/m2 body surface area. However, administration of valbenazine to pregnant rats during organogenesis through lactation produced an increase in the number of stillborn pups and postnatal pup mortalities at doses <1 times the MRHD based on mg/m2 [see Data]. Advise a pregnant woman of the potential risk to a fetus.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. The background risk of major birth defects and miscarriage in the U.S. general population is 2 to 4% and 15 to 20% of clinically recognized pregnancies, respectively.
Data
Animal Data
Valbenazine was administered orally to pregnant rats during the period of organogenesis at 1, 5, and 15 mg/kg/day, which are approximately 0.1, 0.6, and 2 times the MRHD of 80 mg/day based on mg/m2 body surface area. Valbenazine produced a significant decrease in maternal body weight gain at 0.6 and 2 times the MRHD of 80 mg/day based on mg/m2. No adverse embryo fetal effects were produced when valbenazine was administered at doses up to 2 times the MRHD of 80 mg/day based on mg/m2.
Valbenazine was administered orally to pregnant rabbits during the period of organogenesis at 20, 50, and 100 mg/kg/day, which are approximately 5, 12, and 24 times the MRHD of 80 mg/day based on mg/m2. No malformations were observed at doses up to 24 times the MRHD of 80 mg/day based on mg/m2. However, valbenazine produced a delay in fetal development (decreased fetal weights and delayed ossification) at 24 times the MRHD of 80 mg/day based on mg/m2, likely secondary to maternal toxicity (decreased food intake and loss in body weight).
Valbenazine was administered orally to pregnant rats during the period of organogenesis through lactation (day 7 of gestation through day 20 postpartum) at 1, 3, and 10 mg/kg/day, which are approximately 0.1, 0.4, and 1.2 times the MRHD of 80 mg/day based on mg/m2. Valbenazine produced an increase in the incidence of stillbirths and postnatal pup mortality at 0.4 and 1.2 times the MRHD of 80 mg/day based on mg/m2. Valbenazine did not affect neurobehavioral function including learning and memory and had no effect on sexual maturation at doses <1 times the MRHD of 80 mg/day based on mg/m2 (because of death in the majority of the high dose group (1.2 times the MRHD), these parameters were not assessed in this group).
8.2 Lactation
Risk Summary
There is no information regarding the presence of valbenazine or its metabolites in human milk, the effects on the breastfed infant, or the effects on milk production. Valbenazine and its metabolites have been detected in rat milk at concentrations higher than in plasma following oral administration of valbenazine at doses 0.1 to 1.2 times the MRHD based on mg/m2. Based on animal findings of increased perinatal mortality in exposed fetuses and pups, advise a woman not to breastfeed during treatment with INGREZZA or INGREZZA SPRINKLE and for 5 days after the final dose.
8.4 Pediatric Use
Safety and effectiveness of INGREZZA and INGREZZA SPRINKLE have not been established in pediatric patients.
8.5 Geriatric Use
No dose adjustment of INGREZZA or INGREZZA SPRINKLE is required for elderly patients.
Tardive Dyskinesia
In 3 randomized, placebo-controlled studies of INGREZZA in patients with tardive dyskinesia, 16% of patients were 65 years and older. The safety and effectiveness were similar in patients older than 65 years compared to younger patients.
Huntington’s Disease
In the randomized, placebo-controlled study of INGREZZA in 127 patients with chorea associated with Huntington’s disease, 15% were 65 years and older. This study did not include sufficient numbers of subjects aged 65 and older to determine whether they responded differently from younger subjects [see Clinical Studies (14.2)].
8.6 CYP2D6 Poor Metabolizers
Dosage reduction of INGREZZA and INGREZZA SPRINKLE is recommended for known CYP2D6 poor metabolizers [see Dosage and Administration (2.4)]. Increased exposure (Cmax and AUC) to valbenazine’s active metabolite was observed in CYP2D6 poor metabolizers. Increased exposure of active metabolite may increase the risk of exposure-related adverse reactions [see Clinical Pharmacology (12.3, 12.5)].
8.7 Hepatic Impairment
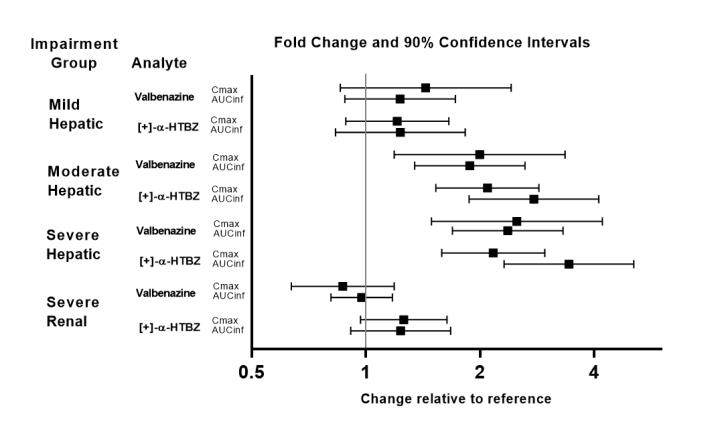
Dosage reduction of INGREZZA and INGREZZA SPRINKLE is recommended for patients with moderate or severe hepatic impairment [see Dosage and Administration (2.3)]. Patients with moderate to severe hepatic impairment (Child-Pugh score 7 to 15) had higher exposure of valbenazine and its active metabolite than patients with normal hepatic function [see Clinical Pharmacology (12.3)].
8.8 Renal Impairment
Dosage adjustment is not necessary for patients with mild, moderate, or severe renal impairment. INGREZZA and INGREZZA SPRINKLE do not undergo primary renal clearance. [see Clinical Pharmacology (12.3)].
-
10 OVERDOSAGE
Human Experience
The pre-marketing clinical trials involving INGREZZA in approximately 850 subjects do not provide information regarding symptoms with overdose.
Management of Overdosage
No specific antidotes for INGREZZA or INGREZZA SPRINKLE are known. In managing overdose, provide supportive care, including close medical supervision and monitoring, and consider the possibility of multiple drug involvement. Consider contacting the Poison Help line (1-800-222-1222) or a medical toxicologist for additional overdose management recommendations.
-
11 DESCRIPTION
INGREZZA and INGREZZA SPRINKLE contains valbenazine, a vesicular monoamine transporter 2 (VMAT2) inhibitor, present as valbenazine tosylate salt, with the chemical name, L-Valine, (2R,3R,11bR)-1,3,4,6,7,11b-hexahydro-9,10-dimethoxy-3-(2-methylpropyl)-2H-benzo[a]quinolizin-2-yl ester, 4-methylbenzenesulfonate (1:2). Valbenazine tosylate is slightly soluble in water. Its molecular formula is C38H54N2O10S2, and its molecular weight is
762.97 g/mol (ditosylate salt) with the following structure:![INGREZZA contains valbenazine, a vesicular monoamine transporter 2 (VMAT2) inhibitor, present as valbenazine tosylate salt, with the chemical name, L-Valine, (2R,3R,11bR)-1,3,4,6,7,11b-hexahydro-9,10-dimethoxy-3-(2-methylpropyl)-2H-benzo[a]quinolizin-2-yl ester, 4-methylbenzenesulfonate (1:2). Valbenazine tosylate is slightly soluble in water. Its molecular formula is C38H54N2O10S2, and its molecular weight is 762.97 g/mol (ditosylate salt) with the following structure:](/dailymed/image.cfm?name=valbenazine-01.jpg&setid=4c970164-cafb-421f-9eb5-c226ef0a3417)
The molecular formula of valbenazine free base is C24H38N2O4 and its molecular weight is 418.57.
INGREZZA is intended for oral administration only. Each capsule contains 73 mg, 109 mg or 146 mg of valbenazine tosylate equivalent to 40 mg, 60 mg or 80 mg of valbenazine free base, respectively. The capsules contain the following inactive ingredients: hypromellose, isomalt, magnesium stearate, pregelatinized starch, and silicified microcrystalline cellulose. The capsule shells contain candurin silver fine, FD&C Blue#1, FD&C Red#40, and gelatin.
INGREZZA SPRINKLE is intended for oral administration. Each capsule contains granules consisting of 73 mg, 109 mg or 146 mg of valbenazine tosylate equivalent to 40 mg, 60 mg or 80 mg of valbenazine free base, respectively. The oral granules contain the following inactive ingredients: silicified microcrystalline cellulose, isomalt, pregelatinized maize starch, hypromellose, magnesium stearate, polyvinyl alcohol, titanium dioxide, polyethylene glycol, and talc in hard gelatin capsules (contains gelatin and candurin silver fine).
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The mechanism of action of valbenazine for the treatment of tardive dyskinesia and chorea in patients with Huntington’s disease is unclear, but is thought to be mediated through the reversible inhibition of vesicular monoamine transporter 2 (VMAT2), a transporter that regulates monoamine uptake from the cytoplasm to the synaptic vesicle for storage and release.
12.2 Pharmacodynamics
Valbenazine inhibits human VMAT2 (Ki ~ 150 nM) with no appreciable binding affinity for VMAT1 (Ki > 10 µM). Valbenazine is converted to the active metabolite [+]-α-dihydrotetrabenazine ([+]-α-HTBZ).
[+]-α-HTBZ also binds with relatively high affinity to human VMAT2 (Ki ~ 3 nM). Valbenazine and [+]-α-HTBZ have no appreciable binding affinity (Ki > 5000 nM) for dopaminergic (including D2), serotonergic (including 5HT2B), adrenergic, histaminergic or muscarinic receptors.Cardiac Electrophysiology
INGREZZA and INGREZZA SPRINKLE may cause an increase in the corrected QT interval in patients who are CYP2D6 poor metabolizers or who are taking a strong CYP2D6 or CYP3A4 inhibitor. An exposure-response analysis of clinical data from two healthy volunteer studies revealed increased QTc interval with higher plasma concentrations of the active metabolite. Based on this model, patients taking an INGREZZA 60 mg or 80 mg dose with increased exposure to the metabolite (e.g., being a CYP2D6 poor metabolizer) may have a mean (upper bound of double-sided 90% CI) QT prolongation of 9.6 (12.0) msec or 11.7 (14.7) msec, respectively as compared to otherwise healthy volunteers given INGREZZA, who had a respective mean (upper bound of double-sided 90% CI) QT prolongation of 5.3 (6.7) msec or 6.7 (8.4) msec [see Warnings and Precautions (5.4)].
12.3 Pharmacokinetics
Valbenazine and its active metabolite ([+]-α-HTBZ) demonstrate approximate proportional increases for the area under the plasma concentration versus time curve (AUC) and maximum plasma concentration (Cmax) after single oral doses from 40 mg to 300 mg (i.e., 50% to 375% of the recommended treatment dose).
Absorption
INGREZZA
Following oral administration of INGREZZA, the time to reach maximum valbenazine plasma concentration (tmax) ranges from 0.5 to 1.0 hours. Valbenazine reaches steady state plasma concentrations within 1 week. The absolute oral bioavailability of valbenazine is approximately 49%. [+]-α-HTBZ gradually forms and reaches Cmax 4 to 8 hours after administration of INGREZZA.
INGREZZA SPRINKLE
Following administration of 80 mg INGREZZA SPRINKLE orally as sprinkle on applesauce, the geometric mean peak plasma concentration (Cmax) of valbenazine was 512 ng/mL, area under the plasma concentration-time curve (AUCinf) was 5,600 ng*hr/mL and the median time to reach Cmax (Tmax) was 1.5 hours. For the [+]-α-HTBZ metabolite, the geometric mean Cmax was 23 ng/mL, AUCinf was 739 ng*hr/mL and the median Tmax was 6 hours.
Following oral administration of 80 mg INGREZZA, the geometric mean Cmax, AUCinf and median Tmax of valbenazine were 744 ng/mL, 6,419 ng*hr/mL and 0.5 hour, respectively. For the [+]-α-HTBZ metabolite, the geometric mean Cmax was 26 ng/mL, AUCinf was 859 ng*hr/mL and the median Tmax was 6 hours.
When 80 mg INGREZZA SPRINKLE capsules were swallowed whole with water, the geometric mean valbenazine Cmax, AUCinf and median Tmax were 685 ng/mL, 5,981 ng*hr/mL and 1 hour, respectively. For the [+]-α-HTBZ metabolite, the geometric mean Cmax was 23 ng/mL, AUCinf was 772 ng*hr/mL and the median Tmax was 6 hours.
Effect of Food
INGREZZA
Ingestion of a high-fat meal decreases valbenazine Cmax by approximately 47% and AUC by approximately 13%. [+]-α-HTBZ Cmax and AUC are unaffected.
INGREZZA SPRINKLE
Ingestion of a high-fat meal decreases valbenazine Cmax by approximately 15% and did not have an appreciable effect on AUC. [+]-α-HTBZ Cmax and AUC are unaffected.
Distribution
The plasma protein binding of valbenazine and [+]-α-HTBZ are greater than 99% and approximately 64%, respectively. The mean steady state volume of distribution of valbenazine is 92 L.
Nonclinical data in Long-Evans rats show that valbenazine can bind to melanin-containing structures of the eye such as the uveal tract. The relevance of this observation to clinical use of INGREZZA or INGREZZA SPRINKLE is unknown.
Elimination
Valbenazine has a mean total plasma systemic clearance value of 7.2 L/hr. Valbenazine and [+]-α-HTBZ have half-lives of 15 to 22 hours.
Metabolism
Valbenazine is extensively metabolized after oral administration by hydrolysis of the valine ester to form the active metabolite ([+]-α-HTBZ) and by oxidative metabolism, primarily by CYP3A4/5, to form mono-oxidized valbenazine and other minor metabolites. [+]-α-HTBZ appears to be further metabolized in part by CYP2D6.
Excretion
Following the administration of a single 50-mg oral dose of radiolabeled C-valbenazine (i.e., ~63% of the recommended treatment dose), approximately 60% and 30% of the administered radioactivity was recovered in the urine and feces, respectively. Less than 2% was excreted as unchanged valbenazine or [+]-α-HTBZ in either urine or feces.
Specific Populations
Exposures of valbenazine in patients with hepatic and severe renal impairment are summarized in Figure 1.
Figure 1: Effects of Hepatic and Severe Renal Impairment on Valbenazine Pharmacokinetics
AUCinf=area under the plasma concentration versus time curve from 0 hours extrapolated to infinity
[+]-α-HTBZ=[+]-α-dihydrotetrabenazine (active metabolite)
Drug Interaction Studies
In Vivo Drug Interactions
The effects of paroxetine, ketoconazole and rifampin on the exposure of valbenazine are summarized in Figure 2.
Figure 2: Effects of Strong CYP2D6 and CYP3A4 Inhibitors and CYP3A4 Inducers on Valbenazine Pharmacokinetics
AUCinf=area under the plasma concentration versus time curve from 0 hours extrapolated to infinity
[+]-α-HTBZ=[+]-α-dihydrotetrabenazine (active metabolite)
The effects of valbenazine on the exposure of other coadministered drugs are summarized in Figure 3.
Figure 3: Effects of Valbenazine on Pharmacokinetics of Other Drugs
AUCinf=area under the plasma concentration versus time curve from 0 hours extrapolated to infinity
In Vitro Drug Interactions
The results of in vitro studies suggest that valbenazine and [+]-α-HTBZ are unlikely to inhibit CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2E1 or CYP3A4/5, or induce CYP1A2, CYP2B6 or CYP3A4/5 at clinically relevant concentrations.
The results of in vitro studies suggest that valbenazine and [+]-α-HTBZ are unlikely to inhibit the transporters (BCRP, OAT1, OAT3, OCT2, OATP1B1, or OATP1B3) at clinically relevant concentrations.
12.5 Pharmacogenomics
CYP2D6 metabolizes the active metabolite of valbenazine ([+]-α-HTBZ). The gene encoding CYP2D6 has polymorphisms that impact protein function. CYP2D6 poor metabolizers are individuals with two non-functioning alleles, resulting in no enzyme activity.
Pharmacokinetic data from CYP2D6 poor metabolizers (n=25) treated with valbenazine demonstrate an approximate 2-fold higher AUCinf and a 1.8-fold higher Cmax of [+]-α-HTBZ compared to normal metabolizers. Dosage reduction is recommended in CYP2D6 poor metabolizers [see Dosage and Administration (2.4), Warnings and Precautions (5.4), and Use in Specific Populations (8.6)].
In a clinical study, AUC of [+]-α-HTBZ was 22% higher and Cmax was 9% lower in intermediate metabolizers (n=7) as compared to normal metabolizers (n=11), which is not considered clinically relevant. The effects of ultrarapid metabolizer status on the pharmacokinetics of [+]-α-HTBZ have not been studied.
Approximately 7% of White populations, 2% of Asian populations, and 2% of African-American populations are poor metabolizers.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Valbenazine did not increase tumors in rats treated orally for 91 weeks at 0.5, 1, and 2 mg/kg/day. These doses are <1 times (0.06, 0.1, and 0.24 times, respectively) the MRHD of 80 mg/day based on mg/m2.
Valbenazine did not increase tumors in hemizygous Tg.rasH2 mice treated orally for 26 weeks at 10, 30 and 75 mg/kg/day, which are 0.6, 1.9 and 4.6 times the MRHD of 80 mg/day based on mg/m2.
Mutagenesis
Valbenazine was not mutagenic in the in vitro bacterial reverse mutation test (Ames) or clastogenic in the in vitro mammalian chromosomal aberrations assay in human peripheral blood lymphocytes or in the in vivo rat bone marrow micronucleus assay.
Impairment of Fertility
In a fertility study, rats were treated orally with valbenazine at 1, 3, and 10 mg/kg/day prior to mating and through mating, for a minimum of 10 weeks (males) or through Day 7 of gestation (females). These doses are 0.1, 0.4, and 1.2 times the MRHD of 80 mg/day based on mg/m2, respectively. Valbenazine delayed mating in both sexes, which led to lower number of pregnancies and disrupted estrous cyclicity at the high dose, 1.2 times the MRHD of 80 mg/day based on mg/m2. Valbenazine had no effects on sperm parameters (motility, count, density) or on uterine parameters (corpora lutea, number of implants, viable implants, pre-implantation loss, early resorptions and post-implantation loss) at any dose.
-
14 CLINICAL STUDIES
14.1 Tardive Dyskinesia
INGREZZA SPRINKLE
The effectiveness of INGREZZA SPRINKLE has been established from adequate and well-controlled studies of INGREZZA for the treatment of tardive dyskinesia. Presented below is a display of the efficacy results of the adequate and well-controlled studies of INGREZZA in patients with tardive dyskinesia.
INGREZZA
A randomized, double-blind, placebo-controlled trial of INGREZZA was conducted in patients with moderate to severe tardive dyskinesia as determined by clinical observation. Patients had underlying schizophrenia, schizoaffective disorder, or a mood disorder. Individuals at significant risk for suicidal or violent behavior and individuals with unstable psychiatric symptoms were excluded.
The Abnormal Involuntary Movement Scale (AIMS) was the primary efficacy measure for the assessment of tardive dyskinesia severity. The AIMS is a 12-item scale; items 1 to 7 assess the severity of involuntary movements across body regions and these items were used in this study. Each of the 7 items was scored on a 0 to 4 scale, rated as: 0=no dyskinesia; 1=low amplitude, present during some but not most of the exam; 2=low amplitude and present during most of the exam (or moderate amplitude and present during some of the exam); 3=moderate amplitude and present during most of exam; or 4=maximal amplitude and present during most of exam. The AIMS dyskinesia total score (sum of items 1 to 7) could thus range from 0 to 28, with a decrease in score indicating improvement. The AIMS was scored by central raters who interpreted the videos blinded to subject identification, treatment assignment, and visit number.
The primary efficacy endpoint was the mean change from baseline in the AIMS dyskinesia total score at the end of Week 6. The change from baseline for two fixed doses of INGREZZA (40 mg or 80 mg) was compared to placebo. At the end of Week 6, subjects initially assigned to placebo were re-randomized to receive INGREZZA 40 mg or 80 mg. Subjects originally randomized to INGREZZA continued INGREZZA at their randomized dose. Follow-up was continued through Week 48 on the assigned drug, followed by a 4-week period off-drug (subjects were not blind to withdrawal).
A total of 234 subjects were enrolled, with 29 (12%) discontinuing prior to completion of the placebo-controlled period. Mean age was 56 (range 26 to 84). Patients were 54% male and 46% female. Patients were 57% Caucasian, 38% African-American, and 5% other. Concurrent diagnoses included schizophrenia/schizoaffective disorder (66%) and mood disorder (34%). With respect to concurrent antipsychotic use, 70% of subjects were receiving atypical antipsychotics, 14% were receiving typical or combination antipsychotics, and 16% were not receiving antipsychotics.
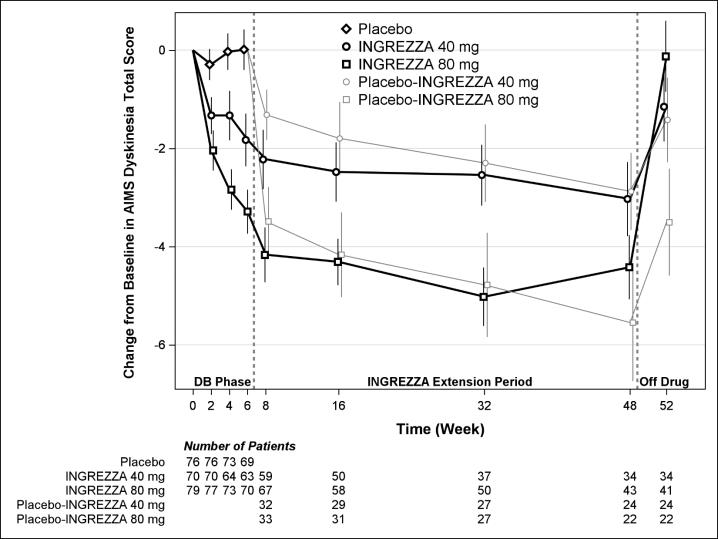
Results are presented in Table 4, with the distribution of responses shown in Figure 4. The change from baseline in the AIMS total dyskinesia score in the 80 mg INGREZZA group was statistically significantly different from the change in the placebo group. Subgroup analyses by gender, age, racial subgroup, underlying psychiatric diagnostic category, and concomitant antipsychotic medication did not suggest any clear evidence of differential responsiveness.
The mean changes in the AIMS dyskinesia total score by visit are shown in Figure 5. Among subjects remaining in the study at the end of the 48-week treatment (N=123 [52.6%]), following discontinuation of INGREZZA, the mean AIMS dyskinesia total score appeared to return toward baseline (there was no formal hypothesis testing for the change following discontinuation).
Table 4: Primary Efficacy Endpoint – Severity of Tardive Dyskinesia at Baseline and the End of Week 6 Endpoint Treatment Group Mean Baseline Score (SD) LS Mean Change from Baseline (SEM)** Placebo-subtracted Difference (95% CI) AIMS Dyskinesia Total Score INGREZZA 40 mg 9.8 (4.1) -1.9 (0.4) -1.8 (-3.0, -0.7) INGREZZA 80 mg* 10.4 (3.6) -3.2 (0.4) -3.1 (-4.2, -2.0) Placebo 9.9 (4.3) -0.1 (0.4) LS Mean=least-squares mean; SD=standard deviation; SEM=standard error of the mean; CI=2-sided 95% confidence interval
*Dose that was statistically significantly different from placebo after adjusting for multiplicity.
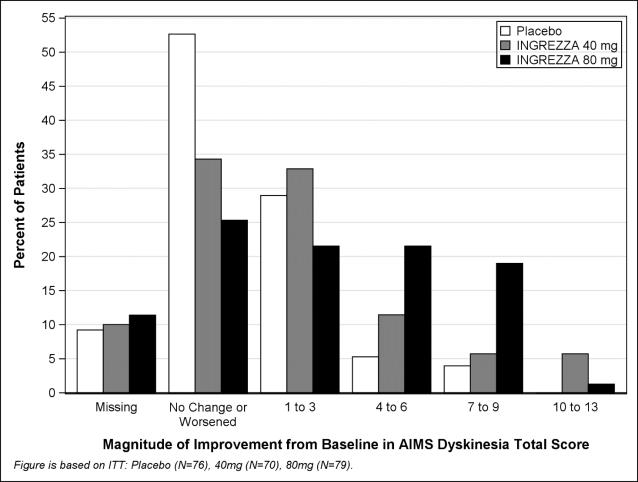
**A negative change from baseline indicates improvement.Figure 4: Percent of Patients with Specified Magnitude of AIMS Total Score Improvement at the End of Week 6
ITT=Intent to Treat; This analysis set includes all randomized patients who had a baseline and at least one post-baseline AIMS dyskinesia total score value reported.
Figure 5: AIMS Dyskinesia Total Score Mean Change from Baseline – Entire Study Duration (Arithmetic Mean)
DB=Double-Blind; After Week 6, subjects initially receiving placebo were re-randomized to receive INGREZZA 40 mg or 80 mg until the end of Week 48. Error bars represent ±1 Standard Error of the Mean (SEM).
Efficacy of INGREZZA 60 mg
Based on modeling and simulation, the predicted mean change from baseline in the AIMS dyskinesia total score at Week 6 for INGREZZA 60 mg once daily in subjects with TD is -2.69 (95% CI: -3.30, -2.13), which is within the efficacy range for INGREZZA 40 mg and 80 mg once daily.
14.2 Chorea Associated with Huntington’s Disease
INGREZZA SPRINKLE
The effectiveness of INGREZZA SPRINKLE has been established from adequate and well-controlled studies of INGREZZA for the treatment of chorea associated with Huntington’s disease. Presented below is a display of the efficacy results of the adequate and well-controlled studies of INGREZZA in patients with chorea associated with Huntington’s disease.
INGREZZA
A randomized, double-blind, placebo-controlled study was conducted to evaluate the efficacy, safety, and tolerability of INGREZZA in patients with chorea associated with Huntington’s disease (NCT04102579). Treatment duration was 12 weeks followed by a 2-week period off drug. INGREZZA was started at 40 mg per day and the dose could be increased every 2 weeks in 20 mg increments up to a maximum dosage of 80 mg per day. The primary efficacy endpoint was the change from baseline to the end of the treatment period (average of Week 10 and Week 12) in the Total Maximal Chorea score of the Unified Huntington’s Disease Rating Scale (UHDRS). The Total Maximal Chorea score is rated from 0 to 4 (with 0 representing no chorea) for 7 different parts of the body, with a total score ranging from 0 to 28.
A total of 128 patients were randomized into the study, and 125 patients were included in the analysis of efficacy. In these patients, the mean age was 54 years (range 25 to 74 years), 46% were male and 96% were White. Greater than 80% of patients were taking the 80 mg daily dosage at the end of the 12-week treatment period.
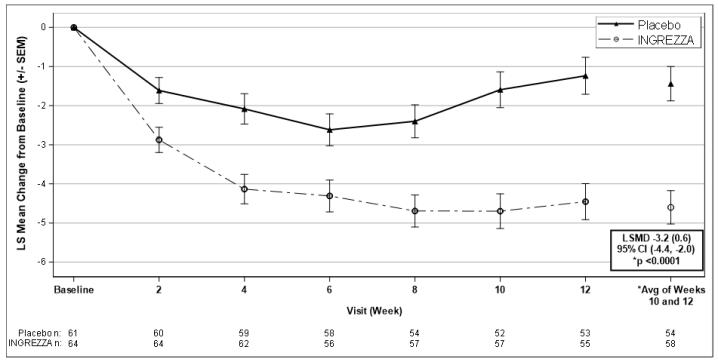
Table 5 and Figure 6 summarize the effects of INGREZZA on chorea based on the Total Maximal Chorea score.
The mean change in Total Maximal Chorea scores for patients receiving INGREZZA improved by 4.6 units (LS mean) from baseline to the end of the treatment period (average of Week 10 and Week 12), compared to 1.4 units in the placebo group. The treatment effect of -3.2 units was statistically significant (p<0.0001) (Figure 6). At the Week 14 follow-up visit (2 weeks after discontinuation of the study medication), the Total Maximal Chorea scores of patients who had received INGREZZA returned to baseline.
Table 5: Primary Efficacy Endpoint - Mean Change from Baseline to End of Treatment in Total Maximal Chorea Score in Patients with Huntington’s Disease Treated with INGREZZA Primary Endpoint Treatment Group Mean Baseline Score (SD) LS Mean Change from Baseline (SEM) b Placebo-subtracted Difference
(95% CI)TMC Scorea INGREZZA
N=6412.2 (2.3) -4.6 (0.4) -3.2 (-4.4, -2.0)
p <0.0001Placebo
N=6112.1 (2.8) -1.4 (0.4) a TMC, Total Maximal Chorea is a subscale of the Unified Huntington’s Disease Rating Scale (UHDRS)
b LS Mean=least-squares mean; SD=standard deviation; SEM=standard error of the mean; CI=2-sided 95% confidence intervalFigure 6: Mean Change in Total Maximal Chorea Score Over Time
LSMD=least-squares mean difference (SEM) [INGREZZA – Placebo]
CI=confidence interval
SEM=standard error of the mean
Figure 7: Distribution of the Change in Total Maximal Chorea Score (Average of Week 10 and 12)
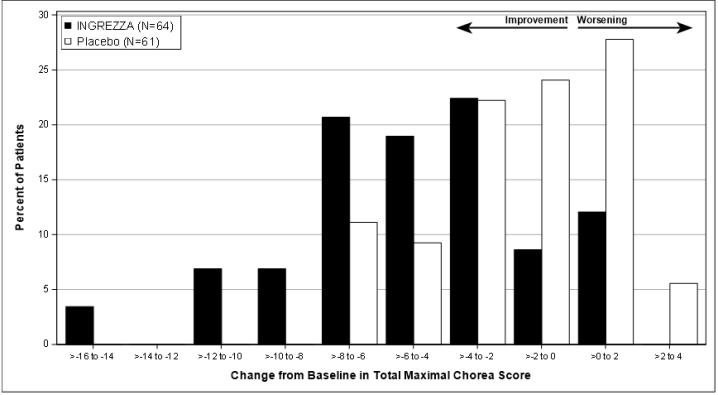
Figure 7 shows the distribution of values for the change in Total Maximal Chorea Score. Negative values indicate a reduction in chorea and positive values indicate an increase in chorea.
In a clinician-rated global impression of change (CGI-C), clinicians rated 43% of patients treated with INGREZZA as “Much Improved” or “Very Much Improved” at the end of treatment, compared to 13% of patients who received placebo (p<0.001).
A patient-rated global impression of change (PGI-C) assessed how patients rated their overall chorea symptoms. Of the patients treated with INGREZZA, 53% rated their symptoms as “Much Improved” or “Very Much Improved” at the end of treatment, compared to 26% of patients who received placebo (p <0.01).
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
INGREZZA (valbenazine) capsules are available as:
40 mg Capsule: White opaque body with a purple cap, printed with ‘VBZ’ and ‘40’ in black ink.
60 mg Capsule: Dark red opaque body with a purple cap, printed with ‘VBZ’ and ‘60’ in black ink.
80 mg Capsule: Purple opaque body and cap, printed with ‘VBZ’ and ‘80’ in black ink.
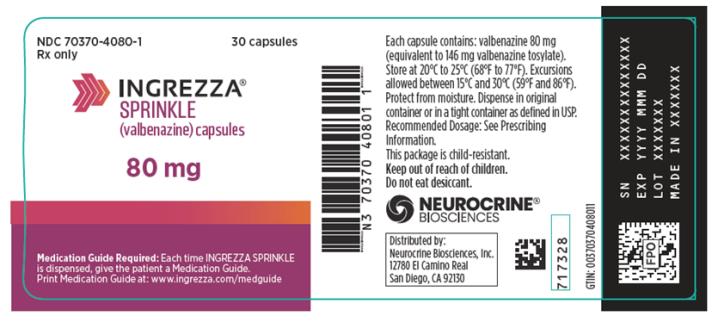
INGREZZA SPRINKLE (valbenazine) capsules are available as:
40 mg Capsule: Pearl white opaque cap and body, printed with a band, directional arrows, and “VBZ 40” in black ink on both the cap and body.
60 mg Capsule: Pearl white opaque cap and body, printed with a band, directional arrows, and “VBZ 60” in dark red ink on both the cap and body.
80 mg Capsule: Pearl white opaque cap and body, printed with a band, directional arrows, and “VBZ 80” in purple ink on both the cap and body.
Table 6: INGREZZA and INGREZZA SPRINKLE Configurations and NDC Numbers
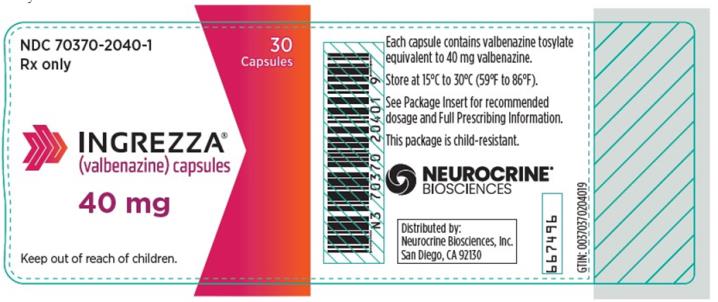
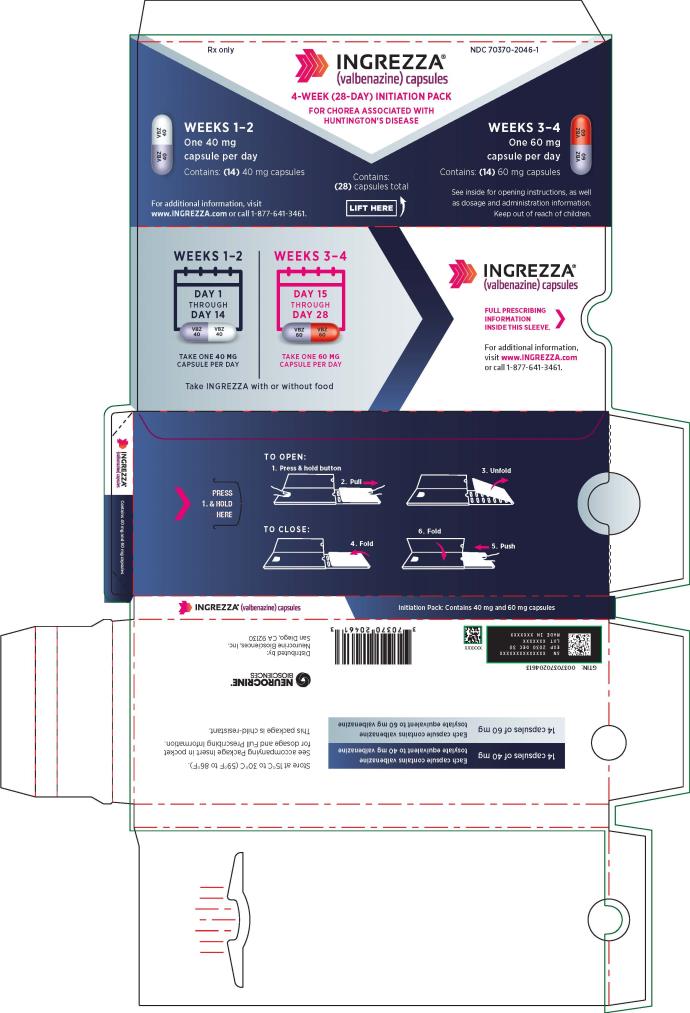
Package Configuration Capsule Strength NDC Number INGREZZA Bottle of 30 40 mg NDC 70370-2040-1 Bottle of 30 60 mg NDC 70370-1060-1 Bottle of 30 80 mg NDC 70370-1080-1 4-week Initiation Pack
for tardive dyskinesia28-day blister pack containing:
7 x 40 mg and 21 x 80 mgNDC 70370-2048-6 4-week Initiation Pack
for chorea associated with Huntington’s disease28-day blister pack containing:
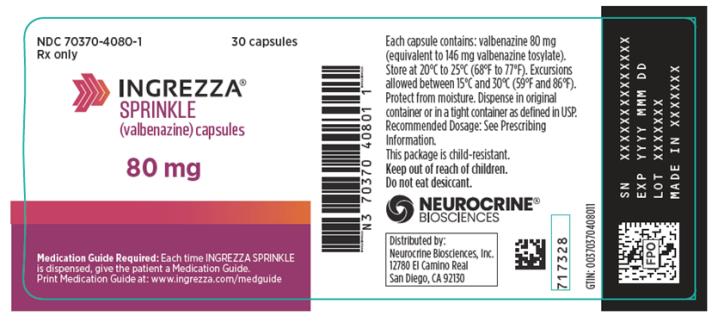
14 x 40 mg and 14 x 60 mgNDC 70370-2046-1 INGREZZA SPRINKLE Bottle of 30 40 mg NDC 70370-4040-1 Bottle of 30 60 mg NDC 70370-4060-1 Bottle of 30 80 mg NDC 70370-4080-1 Storage
INGREZZA: Store at 15°C to 30°C (59°F to 86°F).
INGREZZA SPRINKLE: Store at 20°C to 25°C (68°F to 77°F). Excursions permitted between 15°C and 30°C (59°F and 86°F). Protect from moisture. Dispense in original container or in a tight container as defined in USP.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide and Instructions for Use).
Depression and Suicidal Ideation and Behavior in Patients with Huntington’s Disease
Inform patients, their caregivers, and families of the risks of depression, worsening depression, and suicidal ideation and behavior associated with INGREZZA and INGREZZA SPRINKLE, and instruct them to report behaviors of concern promptly to the treating physician. Patients with Huntington’s disease who express suicidal ideation should be evaluated immediately [see Warnings and Precautions (5.1)].
Hypersensitivity Reactions
Inform patients about the signs and symptoms of hypersensitivity reactions, such as angioedema, including difficulty breathing, swelling of the face, lips, eyelids, tongue or throat. Advise patients to discontinue INGREZZA or INGREZZA SPRINKLE immediately if any of these reactions occur and report to the emergency room if symptoms of angioedema occur [see Warnings and Precautions (5.2)].
Somnolence and Sedation
Inform patients that INGREZZA and INGREZZA SPRINKLE may cause somnolence and may impair the ability to perform tasks that require complex motor and mental skills. Advise patients that until they learn how they respond to INGREZZA or INGREZZA SPRINKLE, they should be careful or avoid doing activities that require them to be alert, such as driving a car or operating machinery [see Warnings and Precautions (5.3)].
Prolongation of the QT Interval
Inform patients to consult their physician immediately if they feel faint, lose consciousness, or have heart palpitations [see Warnings and Precautions (5.4)]. Advise patients to inform physicians that they are taking INGREZZA or INGREZZA SPRINKLE before any new drug is taken.
Neuroleptic Malignant Syndrome (NMS)
Counsel patients about a potentially fatal adverse reaction – neuroleptic malignant syndrome (NMS) – that has been reported in association with administration of VMAT2 inhibitors, including INGREZZA and INGREZZA SPRINKLE. Advise patients to contact a healthcare provider or report to the emergency room if they experience signs or symptoms of NMS [see Warnings and Precautions (5.5)].
Parkinsonism
Inform patients that parkinson-like symptoms may occur while taking INGREZZA or INGREZZA SPRINKLE. Advise patients to consult their healthcare provider if they experience difficulty moving or loss of ability to move muscles voluntarily, tremor, gait disturbances, or drooling [see Warnings and Precautions (5.6)].
Pregnancy
Advise a pregnant patient of the potential risk to a fetus [see Use in Specific Populations (8.1)].
Lactation
Advise a woman not to breastfeed during treatment with INGREZZA or INGREZZA SPRINKLE and for 5 days after the final dose [see Use in Specific Populations (8.2)].
Administration Information for INGREZZA SPRINKLE
Advise patients to read and follow the Instructions for Use for INGREZZA SPRINKLE.
Advise the patient of the following:
- INGREZZA SPRINKLE may be opened and the entire contents of the capsule sprinkled over a bowl containing a small amount (1 tablespoonful) of soft food such as applesauce, yogurt, or pudding. Stir the contents of the capsule into the soft food.
○ Do not sprinkle the contents of the capsule into milk or drinking water.
○ Swallow the drug/food mixture immediately.
○ The mixture may be stored for up to 2 hours at room temperature. Discard any unused portion after 2 hours.
○ Drink a glass (e.g., 240 mL) of water following administration of the drug/food mixture [see Dosage and Administration (2.2)].
- INGREZZA SPRINKLE may also be swallowed whole with water [see Dosage and Administration (2.2)]. Do not crush or chew INGREZZA SPRINKLE.
For further information on INGREZZA or INGREZZA SPRINKLE, call 84-INGREZZA (844-647-3992).
Distributed by:
Neurocrine Biosciences, Inc.
12780 El Camino Real
San Diego, CA 92130INGREZZA is a registered trademark of Neurocrine Biosciences, Inc.
098854011
- INGREZZA SPRINKLE may be opened and the entire contents of the capsule sprinkled over a bowl containing a small amount (1 tablespoonful) of soft food such as applesauce, yogurt, or pudding. Stir the contents of the capsule into the soft food.
-
MEDICATION GUIDE
MEDICATION GUIDE INGREZZA® (in greh' zah)
(valbenazine)
capsulesINGREZZA® SPRINKLE (in greh' zah spring kuhl)
(valbenazine)
capsulesWhat is the most important information I should know about INGREZZA or INGREZZA SPRINKLE?
- INGREZZA or INGREZZA SPRINKLE can cause serious side effects in people with Huntington’s disease, including:
◦ depression
◦ suicidal thoughts
◦ suicidal actions
- Tell your healthcare provider before you start taking INGREZZA or INGREZZA SPRINKLE if you have Huntington’s disease and are depressed (have untreated depression or depression that is not well controlled by medicine) or have suicidal thoughts.
- Pay close attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings. This is especially important when INGREZZA or INGREZZA SPRINKLE is started and when the dose is changed.
- feel sad or have crying spells
- lose interest in seeing your friends or doing things you used to enjoy
- sleep a lot more or a lot less than usual
- feel unimportant
- feel guilty
- feel hopeless or helpless
- feel more irritable, angry or aggressive than usual
- feel more or less hungry than usual or notice a big change in your body weight
- have trouble paying attention
- feel tired or sleepy all the time
- have thoughts about hurting yourself or ending your life
What is INGREZZA or INGREZZA SPRINKLE?
INGREZZA or INGREZZA SPRINKLE is a prescription medicine used to treat adults with:
- movements in the face, tongue, or other body parts that cannot be controlled (tardive dyskinesia).
- the involuntary movements (chorea) of Huntington’s disease. INGREZZA or INGREZZA SPRINKLE does not cure the cause of the involuntary movements, and it does not treat other symptoms of Huntington’s disease, such as problems with thinking or emotions.
Who should not take INGREZZA or INGREZZA SPRINKLE?
Do not take INGREZZA or INGREZZA SPRINKLE if you are allergic to valbenazine, or any of the ingredients in INGREZZA or INGREZZA SPRINKLE. See the end of this Medication Guide for a complete list of ingredients in INGREZZA or INGREZZA SPRINKLE.Before taking INGREZZA or INGREZZA SPRINKLE, tell your healthcare provider about all of your medical conditions, including if you:
- have emotional or mental problems (for example, depression, nervousness, anxiety, anger, agitation, psychosis, previous suicidal thoughts or suicide attempts)
- have liver problems
- have heart disease that is not stable, have heart failure or recently had a heart attack
- have an irregular heart rhythm or heartbeat (QT prolongation, heart arrhythmia)
- are pregnant or plan to become pregnant. INGREZZA or INGREZZA SPRINKLE may harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if INGREZZA or INGREZZA SPRINKLE passes into your breast milk. Do not breastfeed during treatment with INGREZZA or INGREZZA SPRINKLE and for 5 days after the final dose. Talk to your healthcare provider about the best way to feed your baby during treatment with INGREZZA or INGREZZA SPRINKLE.
- take or have taken a monoamine oxidase inhibitor (MAOI) medicine. You should not take INGREZZA or INGREZZA SPRINKLE if you are taking, or have stopped taking, a MAOI within the last 14 days. Ask your healthcare provider if you are not sure if you take a MAOI.
- take digoxin
How should I take INGREZZA or INGREZZA SPRINKLE? - Take INGREZZA or INGREZZA SPRINKLE exactly as your healthcare provider tells you to.
- Your healthcare provider may change your dose of INGREZZA or INGREZZA SPRINKLE during treatment.
- Take INGREZZA or INGREZZA SPRINKLE 1 time each day.
- Take INGREZZA or INGREZZA SPRINKLE with or without food.
-
If you take INGREZZA, swallow the capsule
-
If you take INGREZZA SPRINKLE:
○ You may open the capsule and sprinkle the contents (granules) over a small amount (1 tablespoonful) of soft food such as applesauce, yogurt, or pudding before taking INGREZZA SPRINKLE.
○ You may also swallow the capsule whole with water.
○ Do not add the granules to milk or water.
○ Do not crush or chew INGREZZA SPRINKLE.
○ See the detailed Instructions for Use on how to take a dose of INGREZZA SPRINKLE.
- If you take too much INGREZZA or INGREZZA SPRINKLE, call your healthcare provider or Poison Help line at 1-800-222-1222, or go to the nearest hospital emergency room right away.
What should I avoid while taking INGREZZA or INGREZZA SPRINKLE?
Do not drive a car or operate dangerous machinery until you know how INGREZZA or INGREZZA SPRINKLE affects you because INGREZZA or INGREZZA SPRINKLE can cause sleepiness and tiredness that could cause slow reaction times. Drinking alcohol and taking other medicines that may also cause sleepiness during treatment with INGREZZA or INGREZZA SPRINKLE may increase any sleepiness caused by INGREZZA or INGREZZA SPRINKLE.What are the possible side effects of INGREZZA or INGREZZA SPRINKLE?
INGREZZA or INGREZZA SPRINKLE can cause serious side effects, including:- See “What is the most important information I should know about INGREZZA or INGREZZA SPRINKLE?”
-
Allergic Reactions. Allergic reactions, including an allergic reaction that causes sudden swelling called angioedema, can happen after taking the first dose or after many doses of INGREZZA or INGREZZA SPRINKLE. Signs and symptoms of allergic reactions and angioedema include:
○ trouble breathing or shortness of breath
○ swelling of your face, lips, eyelids, tongue, or throat, or other areas of your skin
○ trouble with swallowing
○ rash, including raised, itchy red areas on your skin (hives)
Swelling in the throat can be life-threatening and can lead to death. Stop taking INGREZZA or INGREZZA SPRINKLE right away and go to the nearest emergency room right away if you develop these signs and symptoms of allergic reactions and angioedema.
-
Sleepiness and tiredness that could cause slow reaction times (somnolence and sedation). See “What should I avoid while taking INGREZZA or INGREZZA SPRINKLE?”
- Heart rhythm problems (QT prolongation). INGREZZA or INGREZZA SPRINKLE may cause a heart rhythm problem known as QT prolongation. You have a higher chance of getting QT prolongation if you also take certain other medicines during treatment with INGREZZA or INGREZZA SPRINKLE. Tell your healthcare provider right away if you develop any signs or symptoms of QT prolongation, including:
◦ fast, slow, or irregular heartbeat (heart palpitations) ◦ shortness of breath ◦ dizziness or lightheadedness ◦ fainting or feeling like you are going to faint. - Neuroleptic Malignant Syndrome (NMS). NMS is a serious condition that can lead to death. Call a healthcare provider right away or go to the nearest emergency room if you develop these signs and symptoms that do not have another obvious cause:
◦ high fever ◦ stiff muscles ◦ problems thinking ◦ irregular pulse or blood pressure ◦ increased sweating ◦ very fast or uneven heartbeat - Parkinson-like symptoms. Tell your healthcare provider if you develop any Parkinson-like symptoms during treatment with INGREZZA or INGREZZA SPRINKLE, including:
◦ body stiffness ◦ drooling ◦ trouble moving or walking ◦ trouble keeping your balance ◦ shaking (tremors) ◦ falls The most common side effect of INGREZZA or INGREZZA SPRINKLE in people with tardive dyskinesia is sleepiness and tiredness that could cause slow reaction times.
The most common side effects of INGREZZA or INGREZZA SPRINKLE in people with chorea associated with Huntington’s disease include sleepiness and tiredness that could cause slow reaction times, raised itchy red areas on your skin (hives), rash, and trouble getting to sleep or staying asleep.
Your doctor may change your dose, temporarily stop, or permanently stop treatment with INGREZZA or INGREZZA SPRINKLE if you develop certain side effects.
These are not all of the possible side effects of INGREZZA or INGREZZA SPRINKLE.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.How should I store INGREZZA or INGREZZA SPRINKLE? - Store INGREZZA at 59°F to 86°F (15°C to 30°C).
- Store INGREZZA SPRINKLE between 68°F to 77°F (20°C to 25°C).
- Keep the bottle tightly closed to protect INGREZZA SPRINKLE from moisture.
- INGREZZA SPRINKLE granules added to soft food can be stored for up to 2 hours at room temperature between 68°F to 77°F (20°C to 25°C). After 2 hours, throw away any unused portion.
- Keep INGREZZA, INGREZZA SPRINKLE and all medicines out of the reach of children.
General information about the safe and effective use of INGREZZA or INGREZZA SPRINKLE.
Medicines are sometimes prescribed for purposes other than those listed in the Medication Guide. Do not use INGREZZA or INGREZZA SPRINKLE for a condition for which it was not prescribed. Do not give INGREZZA or INGREZZA SPRINKLE to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about INGREZZA or INGREZZA SPRINKLE that is written for healthcare professionals.What are the ingredients in INGREZZA or INGREZZA SPRINKLE?
Active ingredient: valbenazine
INGREZZA Inactive ingredients: hypromellose, isomalt, magnesium stearate, pregelatinized starch, and silicified microcrystalline cellulose. The capsule shells contain candurin silver fine, FD&C Blue#1, FD&C Red#40, and gelatin.
INGREZZA SPRINKLE inactive ingredients: silicified microcrystalline cellulose, isomalt, pregelatinized maize starch, hypromellose, magnesium stearate, polyvinyl alcohol, titanium dioxide, polyethylene glycol, and talc. The hard gelatin capsule shells contain gelatin and candurin silver fine.Distributed by: Neurocrine Biosciences, Inc., San Diego, CA 92130, U.S.A.
For more information, go to www.INGREZZA.com or call 84-INGREZZA (844-647-3992).This Medication Guide has been approved by the U.S. Food and Drug Administration Revised: 4/2024
- INGREZZA or INGREZZA SPRINKLE can cause serious side effects in people with Huntington’s disease, including:
-
INSTRUCTIONS FOR USE
INSTRUCTIONS FOR USE
INGREZZA® SPRINKLE (in greh' zah spring kuhl)
(valbenazine)
capsulesThis Instructions for Use contains information on how to take INGREZZA SPRINKLE.
Important Information You Need to Know Before Taking INGREZZA SPRINKLE- You can open the INGREZZA SPRINKLE capsule and sprinkle the contents (granules) on soft food such as applesauce, yogurt, or pudding before you take it.
- You can also swallow the INGREZZA SPRINKLE capsule whole with water with or without food.
-
Do not add the granules to milk or water before you take INGREZZA SPRINKLE.
-
Do not take INGREZZA SPRINKLE through a nasogastric tube (NG Tube), gastrostomy tube (G Tube), or other feeding tube because it may clog the tube.
-
Do not crush or chew INGREZZA SPRINKLE.
- Check to make sure that you have received your prescribed strength of INGREZZA SPRINKLE before taking your dose.
- Check the expiration date on the INGREZZA SPRINKLE bottle. Do not take INGREZZA SPRINKLE if it is expired. Call your healthcare provider or pharmacist if your medicine is expired.
Preparing to Take INGREZZA SPRINKLE
Step 1
Gather your supplies.
You will need (see Figure A):
- The prescribed number of INGREZZA SPRINKLE capsules.
- Soft food such as applesauce, yogurt, or pudding. You will need 1 tablespoonful of soft food.
- A clean tablespoon.
- A clean container such as a small cup or bowl.
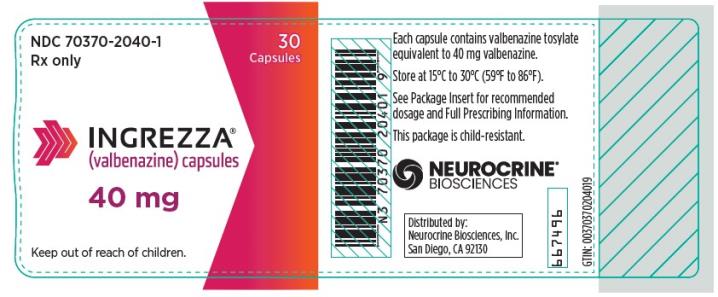
Figure A.Step 2
Wash and dry your hands (see Figure B).

Figure B.Step 3
Place the following items on a clean flat surface:- A tablespoon.
- A container of soft food such as applesauce, yogurt, or pudding.
- The number of capsules you will need for your prescribed dose.
- A small cup or bowl.
Step 4
Place 1 tablespoonful of soft food into a small cup or bowl (see Figure C).
Figure C.Step 5
Opening the INGREZZA SPRINKLE capsule and sprinkling the granules on the soft food
- Hold the INGREZZA SPRINKLE capsule carefully over the soft food (see Figure D).
- Gently pull the INGREZZA SPRINKLE capsule apart, empty the capsule contents (granules) onto the soft food (see Figure E).
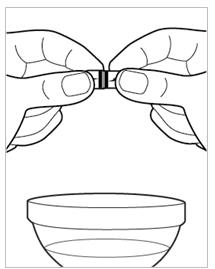
Figure D.
Figure E.- Check the capsule to make sure that you did not miss any granules.
- Tap the capsule to remove any remaining granules.
- Stir the granules into the soft food with the tablespoon (see Figure F).
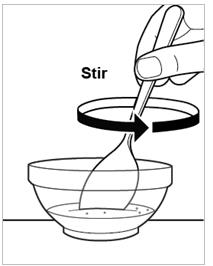
Figure F.Step 6
Taking the soft food and INGREZZA SPRINKLE mixture- Eat all of the food right away after adding the granules.
-
Do not chew the granules.
-
After eating all of the INGREZZA SPRINKLE soft food mixture, drink a glass of water (about 8 ounces or 240 mL) to make sure that all of the medicine is swallowed.
- If you need to, you can store the mixture for up to 2 hours at room temperature between 68°F to 77°F (20°C to 25°C). After 2 hours, throw away any unused portion of the mixture.
Step 7
Cleaning Up and Disposing of INGREZZA SPRINKLE
- Throw away (dispose of) the empty capsule shells in your household trash (see Figure G).
- Wash your hands and all the items used to prepare and take INGREZZA SPRINKLE.
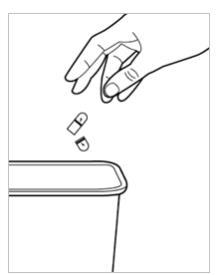
Figure G.Storing INGREZZA SPRINKLE
- Store INGREZZA SPRINKLE between 68°F to 77°F (20°C to 25°C).
- Protect INGREZZA SPRINKLE from moisture to help keep the capsules dry.
- INGREZZA SPRINKLE granules added to soft food can be stored for up 2 hours at room temperature between 68°F to 77°F (20°C to 25°C). After 2 hours, throw away any unused portion.
Distributed by: Neurocrine Biosciences, Inc., San Diego, CA 92130
For more information, go to www.INGREZZA.com or call 84-INGREZZA (844-647-3992).This Instructions for Use has been approved by the U.S. Food and Drug Administration. Approved: 4/2024 - You can open the INGREZZA SPRINKLE capsule and sprinkle the contents (granules) on soft food such as applesauce, yogurt, or pudding before you take it.
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
INGREZZA
valbenazine capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70370-2040 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength VALBENAZINE TOSYLATE (UNII: 5SML1T733B) (VALBENAZINE - UNII:54K37P50KH) VALBENAZINE 40 mg Inactive Ingredients Ingredient Name Strength GELATIN (UNII: 2G86QN327L) FD&C RED NO. 40 (UNII: WZB9127XOA) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) ISOMALT (UNII: S870P55O2W) MAGNESIUM STEARATE (UNII: 70097M6I30) STARCH, CORN (UNII: O8232NY3SJ) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) Product Characteristics Color white, purple Score no score Shape CAPSULE Size 18mm Flavor Imprint Code VBZ;40 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70370-2040-1 30 in 1 BOTTLE; Type 0: Not a Combination Product 12/14/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA209241 12/14/2018 INGREZZA
valbenazine capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70370-1060 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength VALBENAZINE TOSYLATE (UNII: 5SML1T733B) (VALBENAZINE - UNII:54K37P50KH) VALBENAZINE 60 mg Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) GELATIN (UNII: 2G86QN327L) FD&C RED NO. 40 (UNII: WZB9127XOA) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) ISOMALT (UNII: S870P55O2W) MAGNESIUM STEARATE (UNII: 70097M6I30) STARCH, CORN (UNII: O8232NY3SJ) Product Characteristics Color red (Dark Red Body) , purple (Cap) Score no score Shape CAPSULE Size 18mm Flavor Imprint Code VBZ;60 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70370-1060-1 30 in 1 BOTTLE; Type 0: Not a Combination Product 04/23/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA209241 04/23/2021 INGREZZA
valbenazine capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70370-1080 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength VALBENAZINE TOSYLATE (UNII: 5SML1T733B) (VALBENAZINE - UNII:54K37P50KH) VALBENAZINE 80 mg Inactive Ingredients Ingredient Name Strength GELATIN (UNII: 2G86QN327L) FD&C RED NO. 40 (UNII: WZB9127XOA) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) ISOMALT (UNII: S870P55O2W) MAGNESIUM STEARATE (UNII: 70097M6I30) STARCH, CORN (UNII: O8232NY3SJ) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) Product Characteristics Color purple (Purple opaque body and cap) Score no score Shape CAPSULE Size 19mm Flavor Imprint Code VBZ;80 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70370-1080-1 30 in 1 BOTTLE; Type 0: Not a Combination Product 10/04/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA209241 10/04/2017 INGREZZA
valbenazine kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70370-2048 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70370-2048-6 1 in 1 CARTON 12/14/2018 1 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 7 Part 2 21 Part 1 of 2 INGREZZA
valbenazine capsuleProduct Information Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength VALBENAZINE TOSYLATE (UNII: 5SML1T733B) (VALBENAZINE - UNII:54K37P50KH) VALBENAZINE 40 mg Inactive Ingredients Ingredient Name Strength GELATIN (UNII: 2G86QN327L) FD&C RED NO. 40 (UNII: WZB9127XOA) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) ISOMALT (UNII: S870P55O2W) MAGNESIUM STEARATE (UNII: 70097M6I30) STARCH, CORN (UNII: O8232NY3SJ) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) Product Characteristics Color white, purple Score no score Shape CAPSULE Size 18mm Flavor Imprint Code VBZ;40 Contains Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA209241 12/14/2018 Part 2 of 2 INGREZZA
valbenazine capsuleProduct Information Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength VALBENAZINE TOSYLATE (UNII: 5SML1T733B) (VALBENAZINE - UNII:54K37P50KH) VALBENAZINE 80 mg Inactive Ingredients Ingredient Name Strength GELATIN (UNII: 2G86QN327L) FD&C RED NO. 40 (UNII: WZB9127XOA) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) ISOMALT (UNII: S870P55O2W) MAGNESIUM STEARATE (UNII: 70097M6I30) STARCH, CORN (UNII: O8232NY3SJ) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) Product Characteristics Color purple Score no score Shape CAPSULE Size 19mm Flavor Imprint Code VBZ;80 Contains Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA209241 12/14/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA209241 12/14/2018 INGREZZA
valbenazine kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70370-2046 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70370-2046-1 1 in 1 CARTON 08/18/2023 1 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 14 Part 2 14 Part 1 of 2 INGREZZA
valbenazine capsuleProduct Information Item Code (Source) NDC:70370-2040 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength VALBENAZINE TOSYLATE (UNII: 5SML1T733B) (VALBENAZINE - UNII:54K37P50KH) VALBENAZINE 40 mg Inactive Ingredients Ingredient Name Strength GELATIN (UNII: 2G86QN327L) FD&C RED NO. 40 (UNII: WZB9127XOA) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) ISOMALT (UNII: S870P55O2W) MAGNESIUM STEARATE (UNII: 70097M6I30) STARCH, CORN (UNII: O8232NY3SJ) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) Product Characteristics Color white, purple Score no score Shape CAPSULE Size 18mm Flavor Imprint Code VBZ;40 Contains Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA209241 08/18/2023 Part 2 of 2 INGREZZA
valbenazine capsuleProduct Information Item Code (Source) NDC:70370-1060 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength VALBENAZINE TOSYLATE (UNII: 5SML1T733B) (VALBENAZINE - UNII:54K37P50KH) VALBENAZINE 60 mg Inactive Ingredients Ingredient Name Strength GELATIN (UNII: 2G86QN327L) FD&C RED NO. 40 (UNII: WZB9127XOA) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) ISOMALT (UNII: S870P55O2W) MAGNESIUM STEARATE (UNII: 70097M6I30) STARCH, CORN (UNII: O8232NY3SJ) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) Product Characteristics Color purple, red Score no score Shape CAPSULE Size 18mm Flavor Imprint Code VBZ;60 Contains Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA209241 08/18/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA209241 08/18/2023 INGREZZA SPRINKLE
valbenazine capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70370-4040 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength VALBENAZINE TOSYLATE (UNII: 5SML1T733B) (VALBENAZINE - UNII:54K37P50KH) VALBENAZINE 40 mg Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) ISOMALT (UNII: S870P55O2W) STARCH, CORN (UNII: O8232NY3SJ) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) TALC (UNII: 7SEV7J4R1U) GELATIN (UNII: 2G86QN327L) Product Characteristics Color white (Pearl white) Score no score Shape CAPSULE Size 22mm Flavor Imprint Code VBZ;40 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70370-4040-1 30 in 1 BOTTLE; Type 0: Not a Combination Product 04/30/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA218390 04/30/2024 INGREZZA SPRINKLE
valbenazine capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70370-4060 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength VALBENAZINE TOSYLATE (UNII: 5SML1T733B) (VALBENAZINE - UNII:54K37P50KH) VALBENAZINE 60 mg Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) ISOMALT (UNII: S870P55O2W) STARCH, CORN (UNII: O8232NY3SJ) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) TALC (UNII: 7SEV7J4R1U) GELATIN (UNII: 2G86QN327L) Product Characteristics Color white (Pearl white) Score no score Shape CAPSULE Size 22mm Flavor Imprint Code VBZ;60 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70370-4060-1 30 in 1 BOTTLE; Type 0: Not a Combination Product 04/30/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA218390 04/30/2024 INGREZZA SPRINKLE
valbenazine capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70370-4080 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength VALBENAZINE TOSYLATE (UNII: 5SML1T733B) (VALBENAZINE - UNII:54K37P50KH) VALBENAZINE 80 mg Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) ISOMALT (UNII: S870P55O2W) STARCH, CORN (UNII: O8232NY3SJ) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) TALC (UNII: 7SEV7J4R1U) GELATIN (UNII: 2G86QN327L) Product Characteristics Color white (Pearl white) Score no score Shape CAPSULE Size 22mm Flavor Imprint Code VBZ;80 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70370-4080-1 30 in 1 BOTTLE; Type 0: Not a Combination Product 04/30/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA218390 04/30/2024 Labeler - Neurocrine Biosciences, Inc. (800981276)