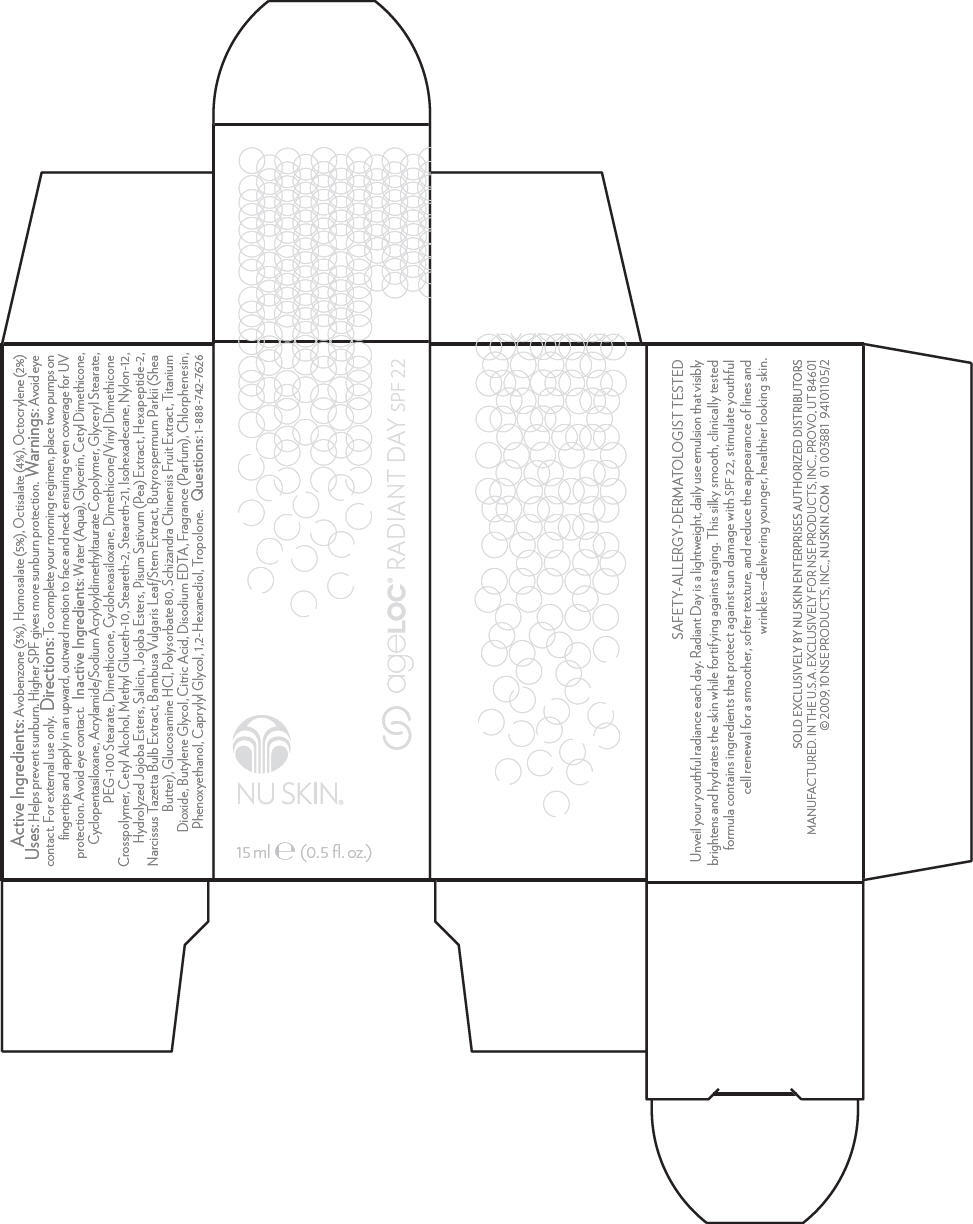
Label: NU SKIN AGELOC RADIANT DAY SPF 22- avobenzone, homosalate, octisalate, and octocrylene lotion
-
Contains inactivated NDC Code(s)
NDC Code(s): 62839-0289-1 - Packager: NSE Products, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated August 13, 2010
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Uses
- Warnings
- Directions
-
Inactive Ingredients
Water (Aqua), Glycerin, Cetyl Dimethicone, Cyclopentasiloxane, Acrylamide/Sodium Acryloyldimethyltaurate Copolymer, Glyceryl Stearate, PEG-100 Stearate, Dimethicone, Cyclohexasiloxane, Dimethicone/Vinyl Dimethicone Crosspolymer, Cetyl Alcohol, Methyl Gluceth-10, Steareth-2, Steareth-21, Isohexadecane, Nylon-12, Hydrolyzed Jojoba Esters, Salicin, Jojoba Esters, Pisum Sativum (Pea) Extract, Hexapeptide-2, Narcissus Tazetta Bulb Extract, Bambusa Vulgaris Leaf/Stem Extract, Butyrospermum Parkii (Shea Butter), Glucosamine HCl, Polysorbate 80, Schizandra Chinensis Fruit Extract, Titanium Dioxide, Butylene Glycol, Citric Acid, Disodium EDTA, Fragrance (Parfum), Chlorphenesin, Phenoxyethanol, Caprylyl Glycol, 1,2-Hexanediol, Tropolone.
- Questions
- PRINCIPAL DISPLAY PANEL - 15 ml Carton
-
INGREDIENTS AND APPEARANCE
NU SKIN AGELOC RADIANT DAY SPF 22
avobenzone, homosalate, octisalate, and octocrylene lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62839-0289 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Avobenzone (UNII: G63QQF2NOX) (Avobenzone - UNII:G63QQF2NOX) Avobenzone 3 mL in 100 mL Homosalate (UNII: V06SV4M95S) (Homosalate - UNII:V06SV4M95S) Homosalate 5 mL in 100 mL Octisalate (UNII: 4X49Y0596W) (Octisalate - UNII:4X49Y0596W) Octisalate 4 mL in 100 mL Octocrylene (UNII: 5A68WGF6WM) (Octocrylene - UNII:5A68WGF6WM) Octocrylene 2 mL in 100 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Glycerin (UNII: PDC6A3C0OX) Cyclomethicone 5 (UNII: 0THT5PCI0R) Glyceryl Monostearate (UNII: 230OU9XXE4) Polyoxyl 100 Stearate (UNII: YD01N1999R) Dimethicone (UNII: 92RU3N3Y1O) Cyclomethicone 6 (UNII: XHK3U310BA) Cetyl Alcohol (UNII: 936JST6JCN) Methyl Gluceth-10 (UNII: N0MWT4C7WH) Steareth-2 (UNII: V56DFE46J5) Steareth-21 (UNII: 53J3F32P58) Isohexadecane (UNII: 918X1OUF1E) Hydrolyzed Jojoba Esters (Acid Form) (UNII: UDR641JW8W) Salicin (UNII: 4649620TBZ) Bambusa Vulgaris Top (UNII: FIW80T6P6V) Shea Butter (UNII: K49155WL9Y) Glucosamine Hydrochloride (UNII: 750W5330FY) Polysorbate 80 (UNII: 6OZP39ZG8H) Titanium Dioxide (UNII: 15FIX9V2JP) Butylene Glycol (UNII: 3XUS85K0RA) Citric Acid Monohydrate (UNII: 2968PHW8QP) Edetate Disodium (UNII: 7FLD91C86K) Chlorphenesin (UNII: I670DAL4SZ) Phenoxyethanol (UNII: HIE492ZZ3T) Caprylyl Glycol (UNII: 00YIU5438U) Tropolone (UNII: 7L6DL16P1T) 1,2-Hexanediol (UNII: TR046Y3K1G) Schisandra Chinensis Fruit (UNII: ABS794681C) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62839-0289-1 1 in 1 CARTON 1 15 mL in 1 BOTTLE, PUMP Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 10/01/2009 Labeler - NSE Products, Inc. (966817975)