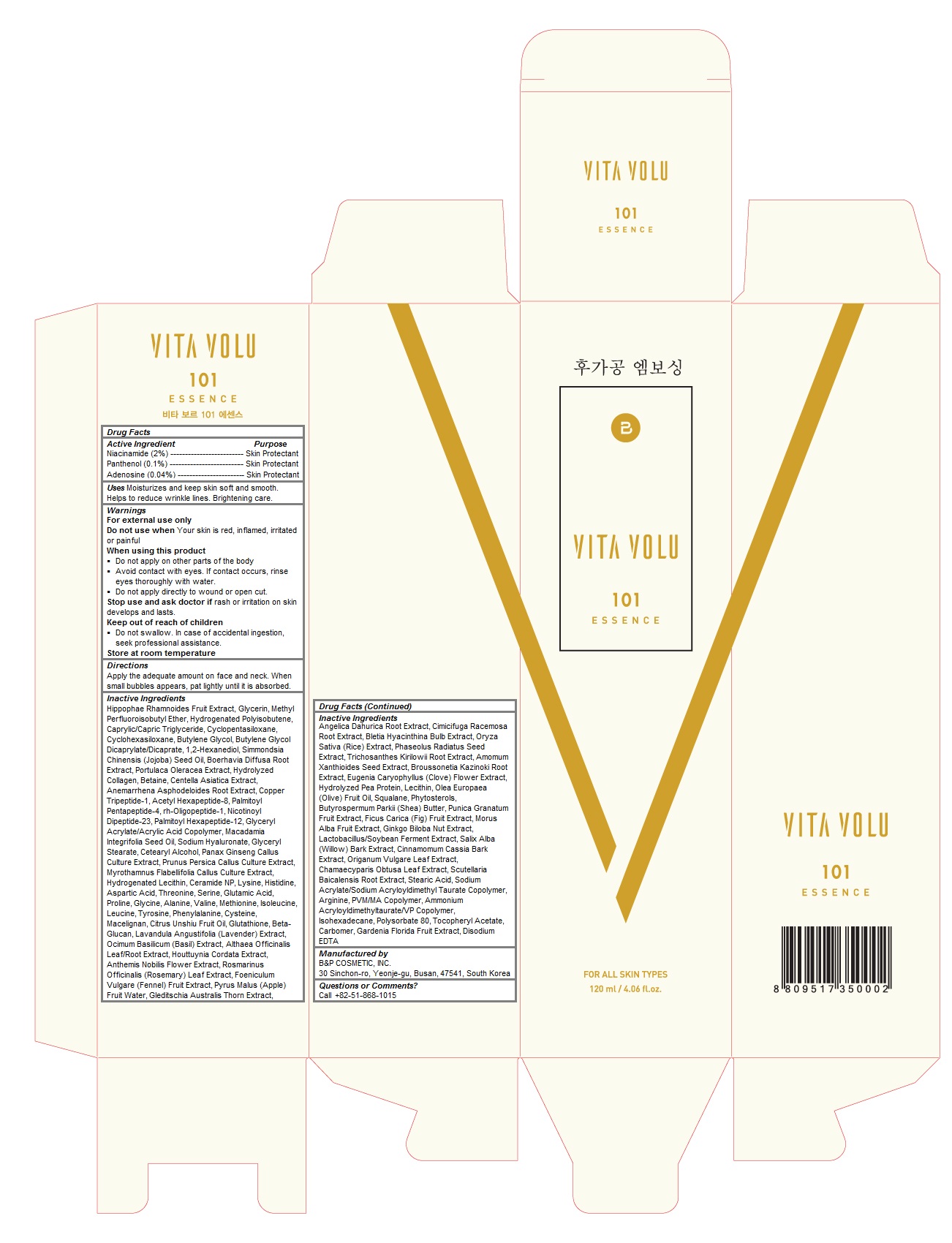
Label: VITA VOLU 101 ESSENCE- niacinamide, panthenol, adenosine liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 71294-101-01, 71294-101-02 - Packager: B&P COSMETIC, INC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated March 3, 2017
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Acitive Ingredients
- Purpose
- Keep out of reach of children
- Uses
-
Warnings
For external use only
Do not use when Your skin is red, inflamed, irritated or painful
When using this product
Do not apply on other parts of the body
Avoid contact with eyes. If contact occurs, rinse eyes thoroughly with water.
Do not apply directly to wound or open cut.
Stop use and ask doctor if rash or irritation on skin develops and lasts.Store at room temperature
- Directions
-
Inactive Ingredients
Hippophae Rhamnoides Fruit Extract, Glycerin, Methyl Perfluoroisobutyl Ether, Hydrogenated Polyisobutene, Caprylic/Capric Triglyceride, Cyclopentasiloxane, Cyclohexasiloxane, Butylene Glycol, Butylene Glycol Dicaprylate/Dicaprate, 1,2-Hexanediol, Simmondsia Chinensis (Jojoba) Seed Oil, Boerhavia Diffusa Root Extract, Portulaca Oleracea Extract, Hydrolyzed Collagen, Betaine, Centella Asiatica Extract, Anemarrhena Asphodeloides Root Extract, Copper Tripeptide-1, Acetyl Hexapeptide-8, Palmitoyl Pentapeptide-4, rh-Oligopeptide-1, Nicotinoyl Dipeptide-23, Palmitoyl Hexapeptide-12, Glyceryl Acrylate/Acrylic Acid Copolymer, Macadamia Integrifolia Seed Oil, Sodium Hyaluronate, Glyceryl Stearate, Cetearyl Alcohol, Panax Ginseng Callus Culture Extract, Prunus Persica Callus Culture Extract, Myrothamnus Flabellifolia Callus Culture Extract, Hydrogenated Lecithin, Ceramide NP, Lysine, Histidine, Aspartic Acid, Threonine, Serine, Glutamic Acid, Proline, Glycine, Alanine, Valine, Methionine, Isoleucine, Leucine, Tyrosine, Phenylalanine, Cysteine, Macelignan, Citrus Unshiu Fruit Oil, Glutathione, Beta-Glucan, Lavandula Angustifolia (Lavender) Extract, Ocimum Basilicum (Basil) Extract, Althaea Officinalis Leaf/Root Extract, Houttuynia Cordata Extract, Anthemis Nobilis Flower Extract, Rosmarinus Officinalis (Rosemary) Leaf Extract, Foeniculum Vulgare (Fennel) Fruit Extract, Pyrus Malus (Apple) Fruit Water, Gleditschia Australis Thorn Extract, Angelica Dahurica Root Extract, Cimicifuga Racemosa Root Extract, Bletia Hyacinthina Bulb Extract, Oryza Sativa (Rice) Extract, Phaseolus Radiatus Seed Extract, Trichosanthes Kirilowii Root Extract, Amomum Xanthioides Seed Extract, Broussonetia Kazinoki Root Extract, Eugenia Caryophyllus (Clove) Flower Extract, Hydrolyzed Pea Protein, Lecithin, Olea Europaea (Olive) Fruit Oil, Squalane, Phytosterols, Butyrospermum Parkii (Shea) Butter, Punica Granatum Fruit Extract, Ficus Carica (Fig) Fruit Extract, Morus Alba Fruit Extract, Ginkgo Biloba Nut Extract, Lactobacillus/Soybean Ferment Extract, Salix Alba (Willow) Bark Extract, Cinnamomum Cassia Bark Extract, Origanum Vulgare Leaf Extract, Chamaecyparis Obtusa Leaf Extract, Scutellaria Baicalensis Root Extract, Stearic Acid, Sodium Acrylate/Sodium Acryloyldimethyl Taurate Copolymer, Arginine, PVM/MA Copolymer, Ammonium Acryloyldimethyltaurate/VP Copolymer, Isohexadecane, Polysorbate 80, Tocopheryl Acetate, Carbomer, Gardenia Florida Fruit Extract, Disodium EDTA
- VITA VOLU 101 ESSENCE
-
INGREDIENTS AND APPEARANCE
VITA VOLU 101 ESSENCE
niacinamide, panthenol, adenosine liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71294-101 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Niacinamide (UNII: 25X51I8RD4) (NIACINAMIDE - UNII:25X51I8RD4) Niacinamide 2.4 g in 120 mL PANTHENOL (UNII: WV9CM0O67Z) (PANTHENOL - UNII:WV9CM0O67Z) PANTHENOL 0.12 g in 120 mL ADENOSINE (UNII: K72T3FS567) (ADENOSINE - UNII:K72T3FS567) ADENOSINE 0.04 g in 120 mL Inactive Ingredients Ingredient Name Strength HIPPOPHAE RHAMNOIDES FRUIT (UNII: AVL0R9111T) Glycerin (UNII: PDC6A3C0OX) METHYL PERFLUOROISOBUTYL ETHER (UNII: HI89P35AAX) HYDROGENATED POLYBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) CYCLOMETHICONE 6 (UNII: XHK3U310BA) Butylene Glycol (UNII: 3XUS85K0RA) BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: 75D21FL1PI) 1,2-Hexanediol (UNII: TR046Y3K1G) JOJOBA OIL (UNII: 724GKU717M) BOERHAVIA DIFFUSA ROOT (UNII: KR0SR09KYL) PURSLANE (UNII: M6S840WXG5) Betaine (UNII: 3SCV180C9W) CENTELLA ASIATICA (UNII: 7M867G6T1U) ANEMARRHENA ASPHODELOIDES ROOT (UNII: U0OC9BJL0I) PREZATIDE COPPER (UNII: 6BJQ43T1I9) ACETYL HEXAPEPTIDE-8 (UNII: L4EL31FWIL) PALMITOYL PENTAPEPTIDE-4 (UNII: KK181SM5JG) NEPIDERMIN (UNII: TZK30RF92W) PALMITOYL HEXAPEPTIDE-12 (UNII: HO4ZT5S86C) MACADAMIA OIL (UNII: 515610SU8C) HYALURONATE SODIUM (UNII: YSE9PPT4TH) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) ASIAN GINSENG (UNII: CUQ3A77YXI) PEACH (UNII: 3OKE88I3QG) MYROTHAMNUS FLABELLIFOLIA WHOLE (UNII: HK3ITD12GB) HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) Ceramide NP (UNII: 4370DF050B) Lysine (UNII: K3Z4F929H6) Histidine (UNII: 4QD397987E) ACETYL ASPARTIC ACID (UNII: 445Y04YIWR) Threonine (UNII: 2ZD004190S) Serine (UNII: 452VLY9402) Glutamic Acid (UNII: 3KX376GY7L) Proline (UNII: 9DLQ4CIU6V) Glycine (UNII: TE7660XO1C) Alanine (UNII: OF5P57N2ZX) Valine (UNII: HG18B9YRS7) Methionine (UNII: AE28F7PNPL) Isoleucine (UNII: 04Y7590D77) Leucine (UNII: GMW67QNF9C) Tyrosine (UNII: 42HK56048U) Phenylalanine (UNII: 47E5O17Y3R) Cysteine (UNII: K848JZ4886) Macelignan (UNII: 8PP3614Z43) CITRUS RETICULATA FRUIT OIL (UNII: 25P9H3QU5E) Glutathione (UNII: GAN16C9B8O) LAVANDULA ANGUSTIFOLIA SUBSP. ANGUSTIFOLIA FLOWERING TOP (UNII: 9YT4B71U8P) OCIMUM BASILICUM FLOWERING TOP (UNII: 7SAB275FP2) ALTHAEA OFFICINALIS LEAF (UNII: E2QQV92338) HOUTTUYNIA CORDATA FLOWERING TOP (UNII: RH041UUZ22) CHAMAEMELUM NOBILE FLOWER (UNII: O2T154T6OG) ROSEMARY (UNII: IJ67X351P9) FENNEL SEED (UNII: G3QC02NIE6) APPLE FRUIT OIL (UNII: 9NT987I3A8) ANGELICA DAHURICA ROOT (UNII: 1V63N2S972) BLACK COHOSH (UNII: K73E24S6X9) BLETILLA STRIATA TUBER (UNII: 00T5SH6SEJ) RICE GERM (UNII: 7N2B70SFEZ) MUNG BEAN (UNII: 1LIB31N73G) TRICHOSANTHES KIRILOWII ROOT (UNII: V409XGE0TS) AMOMUM VILLOSUM SEED (UNII: 745AH91U1C) BROUSSONETIA KAZINOKI ROOT (UNII: 24TET8BDRT) CLOVE (UNII: K48IKT5321) EGG PHOSPHOLIPIDS (UNII: 1Z74184RGV) OLIVE OIL (UNII: 6UYK2W1W1E) Squalane (UNII: GW89575KF9) .BETA.-SITOSTEROL (UNII: S347WMO6M4) SHEA BUTTER (UNII: K49155WL9Y) POMEGRANATE (UNII: 56687D1Z4D) FIG (UNII: TGD87RII2U) WHITE MULBERRY (UNII: MN25R0HH5A) GINKGO BILOBA SEED (UNII: 8CQ734K3W7) SALIX ALBA BARK (UNII: 205MXS71H7) CHINESE CINNAMON (UNII: WS4CQ062KM) OREGANO (UNII: 0E5AT8T16U) CHAMAECYPARIS OBTUSA LEAF (UNII: 7OL154J5XB) SCUTELLARIA BAICALENSIS ROOT (UNII: 7J95K7ID2S) Stearic Acid (UNII: 4ELV7Z65AP) SODIUM ACRYLATE/SODIUM ACRYLOYLDIMETHYLTAURATE COPOLYMER (4000000 MW) (UNII: 1DXE3F3OZX) Arginine (UNII: 94ZLA3W45F) AMMONIUM ACRYLOYLDIMETHYLTAURATE/VP COPOLYMER (UNII: W59H9296ZG) ISOHEXADECANE (UNII: 918X1OUF1E) POLYSORBATE 80 (UNII: 6OZP39ZG8H) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) CARBOXYPOLYMETHYLENE (UNII: 0A5MM307FC) GARDENIA JASMINOIDES FRUIT (UNII: 7CTH8MD549) EDETATE DISODIUM (UNII: 7FLD91C86K) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71294-101-02 1 in 1 PACKAGE 03/03/2017 1 NDC:71294-101-01 120 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 03/02/2017 Labeler - B&P COSMETIC, INC. (689851521) Registrant - B&P COSMETIC, INC. (689851521) Establishment Name Address ID/FEI Business Operations B&P COSMETIC, INC. 689851521 manufacture(71294-101)