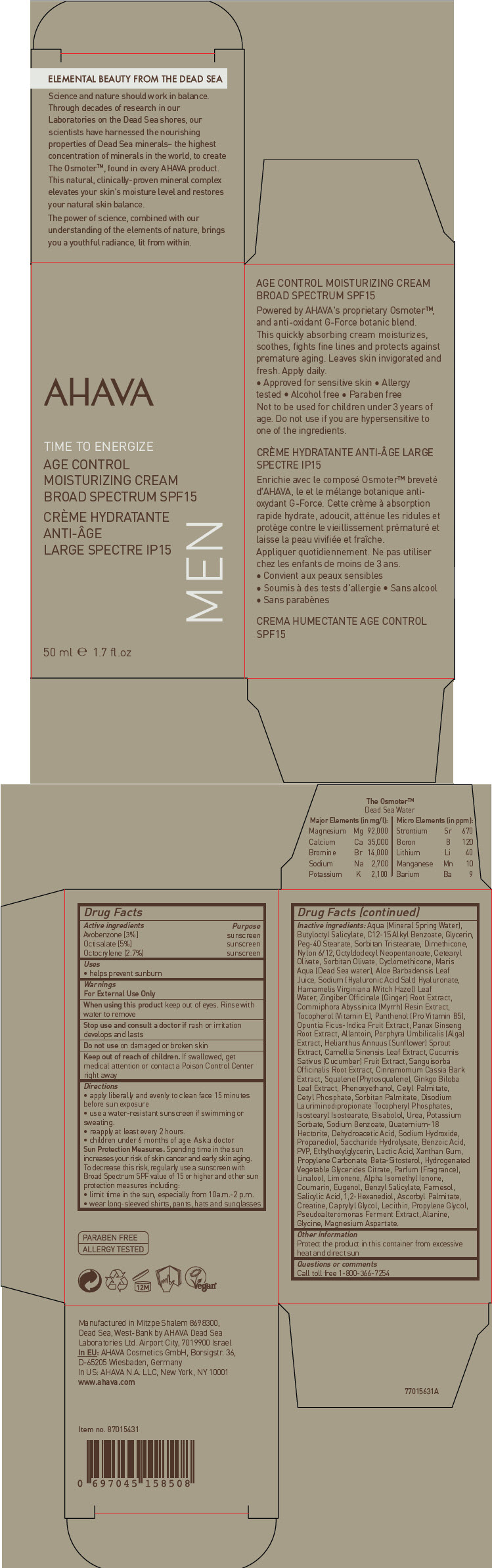
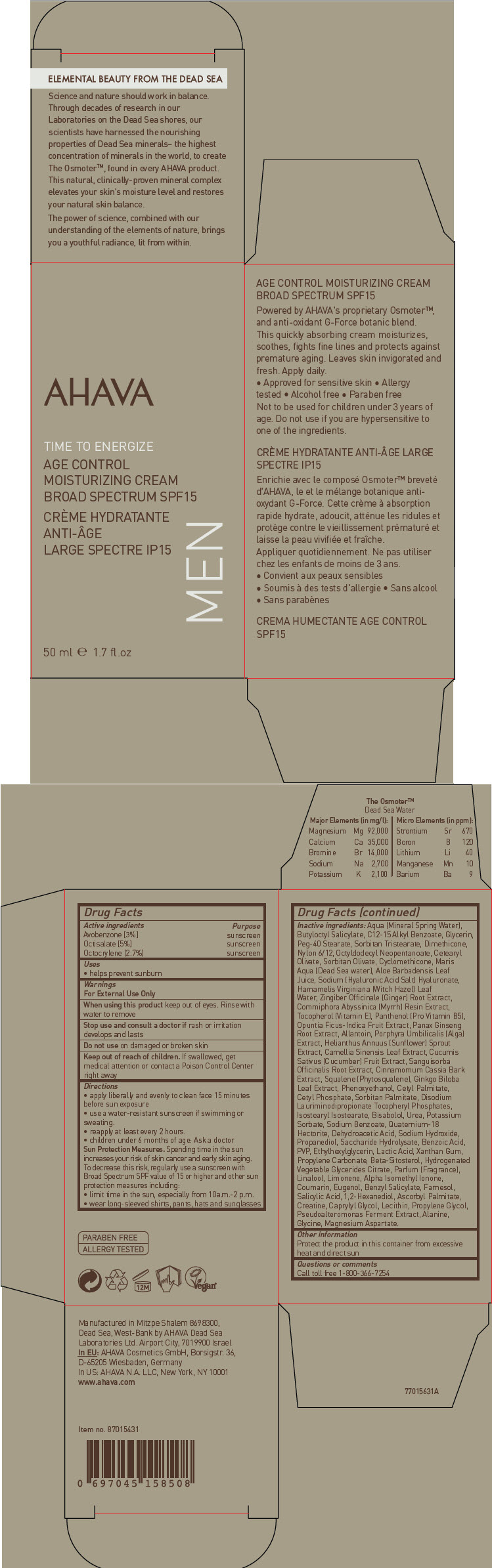
Label: AHAVA ACTIVE DEADSEA MINERALS TIME TO ENERGIZE AGE CONTROL MOISTURIZING BROAD SPECTRUM SPF15- avobenzone, octisalate, and octocrylene cream
- NDC Code(s): 60289-282-24
- Packager: AHAVA Dead Sea Laboratories Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 7, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Uses
- Warnings
-
Directions
- apply liberally and evenly to clean face 15 minutes before sun exposure
- use a water-resistant sunscreen if swimming or sweating.
- reapply at least every 2 hours.
- children under 6 months of age: Ask a doctor
Sun Protection Measures
Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10a.m.-2 p.m.
- wear long-sleeved shirts, pants, hats and sunglasses
-
Inactive ingredients
Aqua (Mineral Spring Water), Butyloctyl Salicylate, C12-15 Alkyl Benzoate, Glycerin, Peg-40 Stearate, Sorbitan Tristearate, Dimethicone, Nylon 6/12, Octyldodecyl Neopentanoate, Cetearyl Olivate, Sorbitan Olivate, Cyclomethicone, Maris Aqua (Dead Sea water), Aloe Barbadensis Leaf Juice, Sodium (Hyaluronic Acid Salt) Hyaluronate, Hamamelis Virginiana (Witch Hazel) Leaf Water, Zingiber Officinale (Ginger) Root Extract, Commiphora Abyssinica (Myrrh) Resin Extract, Tocopherol (Vitamin E), Panthenol (Pro Vitamin B5), Opuntia Ficus-Indica Fruit Extract, Panax Ginseng Root Extract, Allantoin, Porphyra Umbilicalis (Alga) Extract, Helianthus Annuus (Sunflower) Sprout Extract, Camellia Sinensis Leaf Extract, Cucumis Sativus (Cucumber) Fruit Extract, Sanguisorba Officinalis Root Extract, Cinnamomum Cassia Bark Extract, Squalene (Phytosqualene), Ginkgo Biloba Leaf Extract, Phenoxyethanol, Cetyl Palmitate, Cetyl Phosphate, Sorbitan Palmitate, Disodium Lauriminodipropionate Tocopheryl Phosphates, Isostearyl Isostearate, Bisabolol, Urea, Potassium Sorbate, Sodium Benzoate, Quaternium-18 Hectorite, Dehydroacetic Acid, Sodium Hydroxide, Propanediol, Saccharide Hydrolysate, Benzoic Acid, PVP, Ethylhexylglycerin, Lactic Acid, Xanthan Gum, Propylene Carbonate, Beta-Sitosterol, Hydrogenated Vegetable Glycerides Citrate, Parfum (Fragrance), Linalool, Limonene, Alpha Isomethyl Ionone, Coumarin, Eugenol, Benzyl Salicylate, Farnesol, Salicylic Acid, 1,2-Hexanediol, Ascorbyl Palmitate, Creatine, Caprylyl Glycol, Lecithin, Propylene Glycol, Pseudoalteromonas Ferment Extract, Alanine, Glycine, Magnesium Aspartate.
- Other information
- Questions or comments
- PRINCIPAL DISPLAY PANEL - 50 ml Bottle Carton
-
INGREDIENTS AND APPEARANCE
AHAVA ACTIVE DEADSEA MINERALS TIME TO ENERGIZE AGE CONTROL MOISTURIZING BROAD SPECTRUM SPF15
avobenzone, octisalate, and octocrylene creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:60289-282 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Avobenzone (UNII: G63QQF2NOX) (Avobenzone - UNII:G63QQF2NOX) Avobenzone 30 mg in 1 mL Octisalate (UNII: 4X49Y0596W) (Octisalate - UNII:4X49Y0596W) Octisalate 50 mg in 1 mL Octocrylene (UNII: 5A68WGF6WM) (Octocrylene - UNII:5A68WGF6WM) Octocrylene 27 mg in 1 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Alkyl (C12-15) Benzoate (UNII: A9EJ3J61HQ) Butyloctyl Salicylate (UNII: 2EH13UN8D3) Glycerin (UNII: PDC6A3C0OX) Peg-40 Stearate (UNII: ECU18C66Q7) Sorbitan Tristearate (UNII: 6LUM696811) Octyldodecyl Neopentanoate (UNII: X8725R883T) Dimethicone (UNII: 92RU3N3Y1O) Sorbitan Olivate (UNII: MDL271E3GR) Cetearyl Olivate (UNII: 58B69Q84JO) Cyclomethicone (UNII: NMQ347994Z) Phenoxyethanol (UNII: HIE492ZZ3T) Cetyl Palmitate (UNII: 5ZA2S6B08X) Sorbitan Monopalmitate (UNII: 77K6Z421KU) Propylene Carbonate (UNII: 8D08K3S51E) Quaternium-18 Hectorite (UNII: IIS3YBV1XX) Ethylhexylglycerin (UNII: 147D247K3P) Caprylyl Glycol (UNII: 00YIU5438U) Hyaluronate Sodium (UNII: YSE9PPT4TH) Aloe Vera Leaf (UNII: ZY81Z83H0X) Cetyl Phosphate (UNII: VT07D6X67O) Isostearyl Isostearate (UNII: IV0Z586Z4Y) Cucumber (UNII: YY7C30VXJT) Alanine (UNII: OF5P57N2ZX) Creatine (UNII: MU72812GK0) Glycine (UNII: TE7660XO1C) Magnesium Aspartate (UNII: R17X820ROL) Invert Sugar (UNII: ED959S6ACY) Urea (UNII: 8W8T17847W) Disodium Lauriminodipropionate Tocopheryl Phosphates (UNII: 0K5Y9U1P6M) Propanediol (UNII: 5965N8W85T) Chinese Cinnamon (UNII: WS4CQ062KM) Sanguisorba Officinalis Root (UNII: 4NYV2HT01X) Levomenol (UNII: 24WE03BX2T) Xanthan Gum (UNII: TTV12P4NEE) Allantoin (UNII: 344S277G0Z) Ginger (UNII: C5529G5JPQ) Ginkgo (UNII: 19FUJ2C58T) Green Tea Leaf (UNII: W2ZU1RY8B0) Asian Ginseng (UNII: CUQ3A77YXI) Panthenol (UNII: WV9CM0O67Z) Hamamelis Virginiana Top Water (UNII: NT00Y05A2V) Ascorbyl Palmitate (UNII: QN83US2B0N) .Beta.-Sitosterol (UNII: S347WMO6M4) Propylene Glycol (UNII: 6DC9Q167V3) Squalene (UNII: 7QWM220FJH) Tocopherol (UNII: R0ZB2556P8) Porphyra Umbilicalis (UNII: 14AN0J70WO) Prickly Pear Fruit (UNII: 18V8PAQ629) Helianthus Annuus Sprout (UNII: 4P26HG1S5W) Salicylic Acid (UNII: O414PZ4LPZ) Commiphora Madagascariensis Resin (UNII: WCM0X628RY) Isomethyl-.Alpha.-Ionone (UNII: 9XP4LC555B) Benzyl Salicylate (UNII: WAO5MNK9TU) Coumarin (UNII: A4VZ22K1WT) Eugenol (UNII: 3T8H1794QW) Linalool, (+/-)- (UNII: D81QY6I88E) Povidone K30 (UNII: U725QWY32X) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) SODIUM HYDROXIDE (UNII: 55X04QC32I) LIMONENE, (-)- (UNII: 47MAJ1Y2NE) FARNESOL, (2E,6Z)- (UNII: 413TVZ4919) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) Lecithin, Soybean (UNII: 1DI56QDM62) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:60289-282-24 1 in 1 CARTON 07/25/2013 1 50 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M020 07/25/2013 Labeler - AHAVA Dead Sea Laboratories Ltd (600056907)