Label: RANOLAZINE tablet, extended release
- NDC Code(s): 63304-017-05, 63304-017-28, 63304-017-60, 63304-018-05, view more
- Packager: Sun Pharmaceutical Industries, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated August 17, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use RANOLAZINE EXTENDED-RELEASE TABLETS safely and effectively. See full prescribing information for RANOLAZINE EXTENDED-RELEASE ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE Ranolazine extended-release tablets are indicated for the treatment of chronic angina. Ranolazine extended-release tablets may be used with beta-blockers, nitrates, calcium channel blockers ...
-
2 DOSAGE AND ADMINISTRATION 2.1 Dosing Information - Initiate ranolazine extended-release tablets dosing at 500 mg twice daily and increase to 1000 mg twice daily, as needed, based on clinical symptoms. Take ranolazine ...
-
3 DOSAGE FORMS AND STRENGTHS Ranolazine extended-release tablets are supplied as film-coated, oblong-shaped, extended-release tablets in the following strengths: • 500 mg tablets are light orange colored, debossed with ...
-
4 CONTRAINDICATIONS Ranolazine is contraindicated in patients: • Taking strong inhibitors of CYP3A [see Drug Interactions (7.1)] • Taking inducers of CYP3A [see Drug Interactions (7.1)] • With liver cirrhosis [see ...
-
5 WARNINGS AND PRECAUTIONS 5.1 QT Interval Prolongation - Ranolazine blocks IKr and prolongs the QTc interval in a dose-related manner. Clinical experience in an acute coronary syndrome population did not show an ...
-
6 ADVERSE REACTIONS 6.1 Clinical Trial Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 DRUG INTERACTIONS 7.1 Effects of Other Drugs on Ranolazine - Strong CYP3A Inhibitors - Do not use ranolazine with strong CYP3A inhibitors, including ketoconazole, itraconazole, clarithromycin, nefazodone ...
-
8 USE IN SPECIFIC POPULATIONS 8.1 Pregnancy - Risk Summary - There are no available data on ranolazine use in pregnant women to inform any drug-associated risks. Studies in rats and rabbits showed no evidence of fetal harm ...
-
10 OVERDOSAGE Hypotension, QT prolongation, bradycardia, myoclonic activity, severe tremor, unsteady gait/incoordination, dizziness, nausea, vomiting, dysphasia, and hallucinations have been seen in cases of ...
-
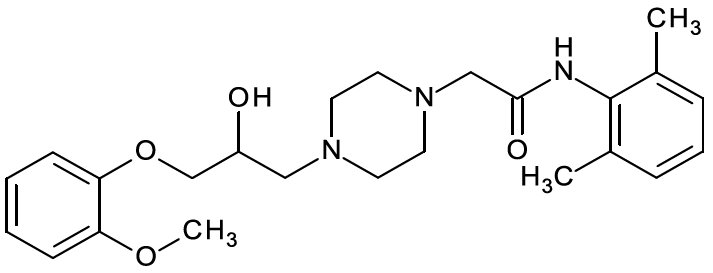
11 DESCRIPTION Ranolazine tablets are available as film-coated, non-scored, extended-release tablets for oral administration. Ranolazine is a racemic mixture, chemically described as 1-piperazineacetamide ...
-
12 CLINICAL PHARMACOLOGY 12.1 Mechanism of Action - The mechanism of action of ranolazine’s antianginal effects has not been determined. Ranolazine has anti-ischemic and antianginal effects that do not depend upon ...
-
13 NONCLINICAL TOXICOLOGY 13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Ranolazine tested negative for genotoxic potential in the following assays: Ames bacterial mutation assay, Saccharomyces assay for ...
-
14 CLINICAL STUDIES 14.1 Chronic Stable Angina - CARISA (Combination Assessment of Ranolazine In Stable Angina) was a study in 823 chronic angina patients randomized to receive 12 weeks of treatment with ...
-
15 REFERENCES M.A. Suckow et al. The anti-ischemia agent ranolazine promotes the development of intestinal tumors in APC (min/+) mice. Cancer Letters 209 (2004) :165−9.
-
16 HOW SUPPLIED/STORAGE AND HANDLING Ranolazine extended-release tablets are supplied as film-coated, oblong-shaped, extended-release tablets in the following strengths: • 500 mg tablets are light orange colored, debossed with ...
-
17 PATIENT COUNSELING INFORMATION Advise the patient to read the FDA-approved patient labeling (Patient Information). Inform patients that ranolazine extended-release tablets will not abate an acute angina episode. Strong CY3PA ...
-
Patient Information Ranolazine (ran-OH-lah-zeen) extended-release tablets - Dosing Strengths: 500 mg tablets - 1000 mg tablets - Rx only - Read this Patient Information before you start taking ranolazine ...
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL NDC 63304-017-60 - Ranolazine Extended-Release Tablets - 500 mg - Swallow ranolazine extended-release tablets whole; do not crush, break, or chew. Rx only - 60 Tablets - SUN PHARMA
-
Package/Label Display Panel NDC 63304-018-60 - Ranolazine Extended-Release Tablets - 1000 mg - Swallow ranolazine extended-release tablets whole; do not crush, break, or chew. Rx only - 60 Tablets - SUN PHARMA
-
INGREDIENTS AND APPEARANCEProduct Information