Label: PHARMASKINCARE ACNECARE EXFOLIATOR- salicylic acid solution
- NDC Code(s): 68062-9110-1
- Packager: Spa de Soleil
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated October 11, 2017
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Warnings
-
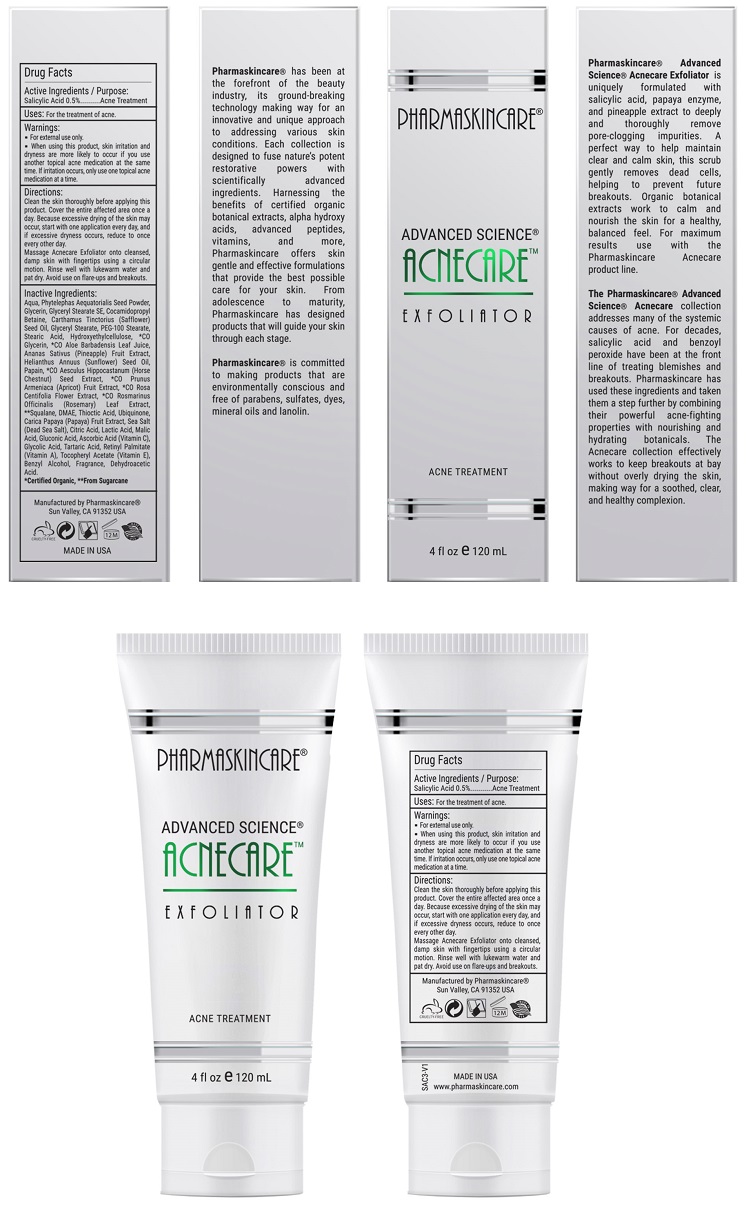
Directions
Clean the skin thoroughly before applying this product. Cover the entire affected area with a thin layer and rinse thoroughly one to three times daily. Because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor.
Massage Acnecare Exfoliator onto cleansed, damp skin with fingertips using a circular motion. Rinse well with lukewarm water and pat dry. Avoid use on flare-ups and breakouts.
- Uses:
-
Inactive Ingredients:
Water, Phytelephas Aequatorialis Seed Powder, Glycerin, Glyceryl Stearate SE, Cocamidopropyl Betaine, Carthamus Tinctorius (Safflower) Seed Oil, Glyceryl Stearate, PEG-100 Stearate, Stearic Acid, Hydroxyethylcellulose, *CO Glycerin, *CO Aloe Barbadensis Leaf Juice, Ananas Sativus (Pineapple) Fruit Extract, Helianthus Annuus (Sunflower) Seed Oil, Papain, *CO Aesculus Hippocastanum (Horse Chestnut) Seed Extract, *CO Prunus Armeniaca (Apricot) Fruit Extract, *CO Rosa Centifolia Flower Extract, *CO Rosmarinus Officinalis (Rosemary) Leaf Extract, **Squalane, DMAE, Thioctic Acid, Ubiquinone, Carica Papaya (Papaya) Fruit Extract, Sea Salt (Dead Sea Salt), Malus Domestica Fruit Cell Culture Extract, Xanthan Gum, Citric Acid, Lactic Acid, Malic Acid, Gluconic Acid, Ascorbic Acid (Vitamin C), Lecithin, Glycolic Acid, Tartaric Acid, Tocopheryl Acetate (Vitamin E), Retinyl Palmitate (Vitamin A), Benzyl Alcohol, Fragrance, Dehydroacetic Acid
- Warning
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PHARMASKINCARE ACNECARE EXFOLIATOR
salicylic acid solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68062-9110 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 0.013 mg in 2.5 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) SAFFLOWER OIL (UNII: 65UEH262IS) PHYTELEPHAS AEQUATORIALIS SEED (UNII: 2DJN5A6ONH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68062-9110-1 120 mL in 1 TUBE; Type 0: Not a Combination Product 10/10/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333D 10/10/2017 Labeler - Spa de Soleil (874682867) Establishment Name Address ID/FEI Business Operations Spa de Soleil 874682867 manufacture(68062-9110)