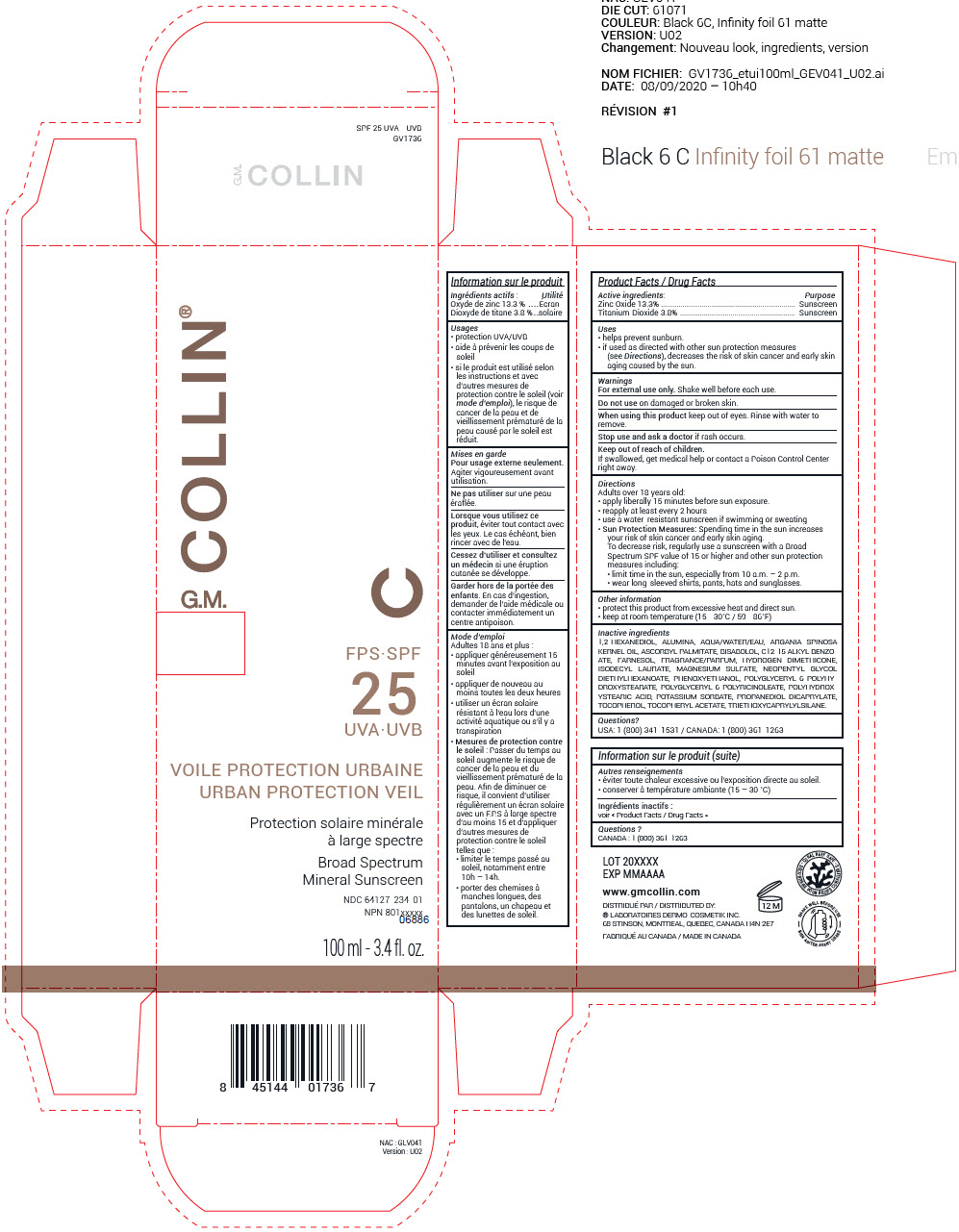
Label: GM COLLIN SPF 25 UVA - UVB URBAN PROTECTION VEIL SUNSCREEN- zinc oxide and titanium dioxide cream
- NDC Code(s): 64127-234-01
- Packager: Laboratoires Dermo-Cosmetik Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 27, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
-
Uses
- helps prevent sunburn.
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
- Warnings
-
Directions
Adults over 18 years old:
- apply liberally 15 minutes before sun exposure.
- reapply at least every 2 hours
- use a water-resistant sunscreen if swimming or sweating
-
Sun Protection Measures: Spending time in the sun increases your risk of skin cancer and early skin aging.
To decrease risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:- limit time in the sun, especially from 10 a.m. – 2 p.m.
- wear long-sleeved shirts, pants, hats and sunglasses.
- Other information
-
Inactive ingredients
1,2-HEXANEDIOL, ALUMINA, AQUA/WATER/EAU, ARGANIA SPINOSA KERNEL OIL, ASCORBYL PALMITATE, BISABOLOL, C12-15 ALKYL BENZOATE, FARNESOL, FRAGRANCE/PARFUM, HYDROGEN DIMETHICONE, ISODECYL LAURATE, MAGNESIUM SULFATE, NEOPENTYL GLYCOL DIETHYLHEXANOATE, PHENOXYETHANOL, POLYGLYCERYL-6 POLYHYDROXYSTEARATE, POLYGLYCERYL-6 POLYRICINOLEATE, POLYHYDROXYSTEARIC ACID, POTASSIUM SORBATE, PROPANEDIOL DICAPRYLATE, TOCOPHEROL, TOCOPHERYL ACETATE, TRIETHOXYCAPRYLYLSILANE.
- Questions?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 100 ml Bottle Box
-
INGREDIENTS AND APPEARANCE
GM COLLIN SPF 25 UVA - UVB URBAN PROTECTION VEIL SUNSCREEN
zinc oxide and titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:64127-234 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 14.6 mg in 100 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 4.2 mg in 100 mL Inactive Ingredients Ingredient Name Strength 1,2-HEXANEDIOL (UNII: TR046Y3K1G) ALUMINUM OXIDE (UNII: LMI26O6933) WATER (UNII: 059QF0KO0R) ARGAN OIL (UNII: 4V59G5UW9X) ASCORBYL PALMITATE (UNII: QN83US2B0N) LEVOMENOL (UNII: 24WE03BX2T) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) FARNESOL (UNII: EB41QIU6JL) ISODECYL LAURATE (UNII: 254BX4O0JU) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) NEOPENTYL GLYCOL DIETHYLHEXANOATE (UNII: U68ZV6W62C) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYGLYCERYL-6 POLYRICINOLEATE (UNII: YPM0ZOC2HR) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) PROPANEDIOL DICAPRYLATE (UNII: C577OMC6UH) TOCOPHEROL (UNII: R0ZB2556P8) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:64127-234-01 1 in 1 BOX 01/15/2021 1 100 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 01/15/2021 Labeler - Laboratoires Dermo-Cosmetik Inc. (249335480) Establishment Name Address ID/FEI Business Operations Dermolab Pharma Ltd 245414743 manufacture(64127-234)