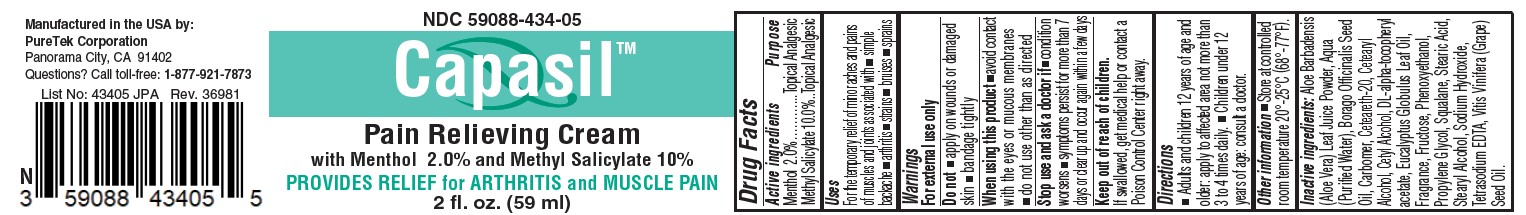
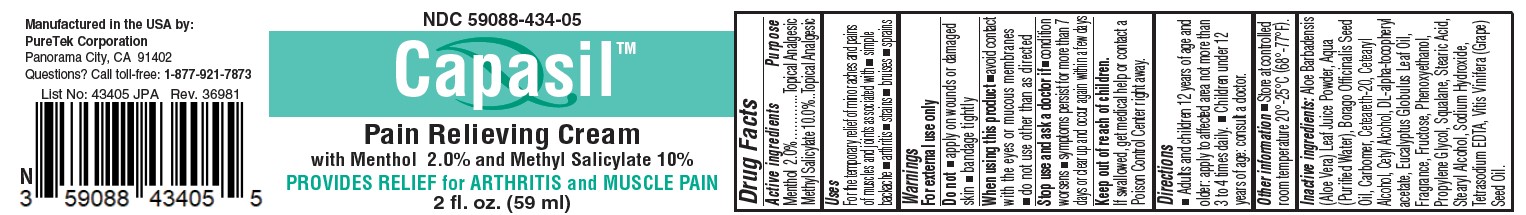
Label: CAPASIL PAIN RELIEVING- menthol, methyl salicylate cream
- NDC Code(s): 59088-434-05
- Packager: PureTek Corporation
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 1, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Purpose
- Keep out of reach of children.
- Uses
- Warnings
- Directions
-
Inactive ingredients
Aloe Barbadensis(Aloe Vera) Leaf Juice Powder, Aqua (Purified Water), Borago Officinalis Seed
Oil, Carbomer, Ceteareth-20, Cetearyl Alcohol, Cetyl Alcohol, DL-alpha-tocopheryl
acetate, Eucalyptus Globulus Leaf Oil, Fragrance, Fructose, Phenoxyethanol,
Propylene Glycol, Squalane, Stearic Acid, Stearyl Alcohol, Sodium Hydroxide,
Tetrasodium EDTA, Vitis Vinifera (Grape) Seed Oil. - STORAGE AND HANDLING
- Label
-
INGREDIENTS AND APPEARANCE
CAPASIL PAIN RELIEVING
menthol, methyl salicylate creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59088-434 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METHYL SALICYLATE (UNII: LAV5U5022Y) (SALICYLIC ACID - UNII:O414PZ4LPZ) METHYL SALICYLATE 100 mg in 1 mL MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength MENHADEN OIL (UNII: 1D8HWC57D0) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) AMINOMETHYL PROPANEDIOL (UNII: CZ7BU4QZJZ) LIMONENE, (+)- (UNII: GFD7C86Q1W) PALMITIC ACID (UNII: 2V16EO95H1) POLYOXYL 20 CETOSTEARYL ETHER (UNII: YRC528SWUY) ALOE VERA LEAF (UNII: ZY81Z83H0X) BORAGE SEED OIL (UNII: F8XAG1755S) CARBOMER HOMOPOLYMER TYPE B (ALLYL SUCROSE CROSSLINKED) (UNII: Z135WT9208) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) CETYL ALCOHOL (UNII: 936JST6JCN) EUCALYPTUS OIL (UNII: 2R04ONI662) FRUCTOSE (UNII: 6YSS42VSEV) PHENOXYETHANOL (UNII: HIE492ZZ3T) WATER (UNII: 059QF0KO0R) SQUALANE (UNII: GW89575KF9) STEARIC ACID (UNII: 4ELV7Z65AP) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) EDETATE SODIUM (UNII: MP1J8420LU) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) GRAPE SEED OIL (UNII: 930MLC8XGG) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59088-434-05 59 mL in 1 JAR; Type 0: Not a Combination Product 10/14/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M018 10/14/2020 Labeler - PureTek Corporation (785961046)