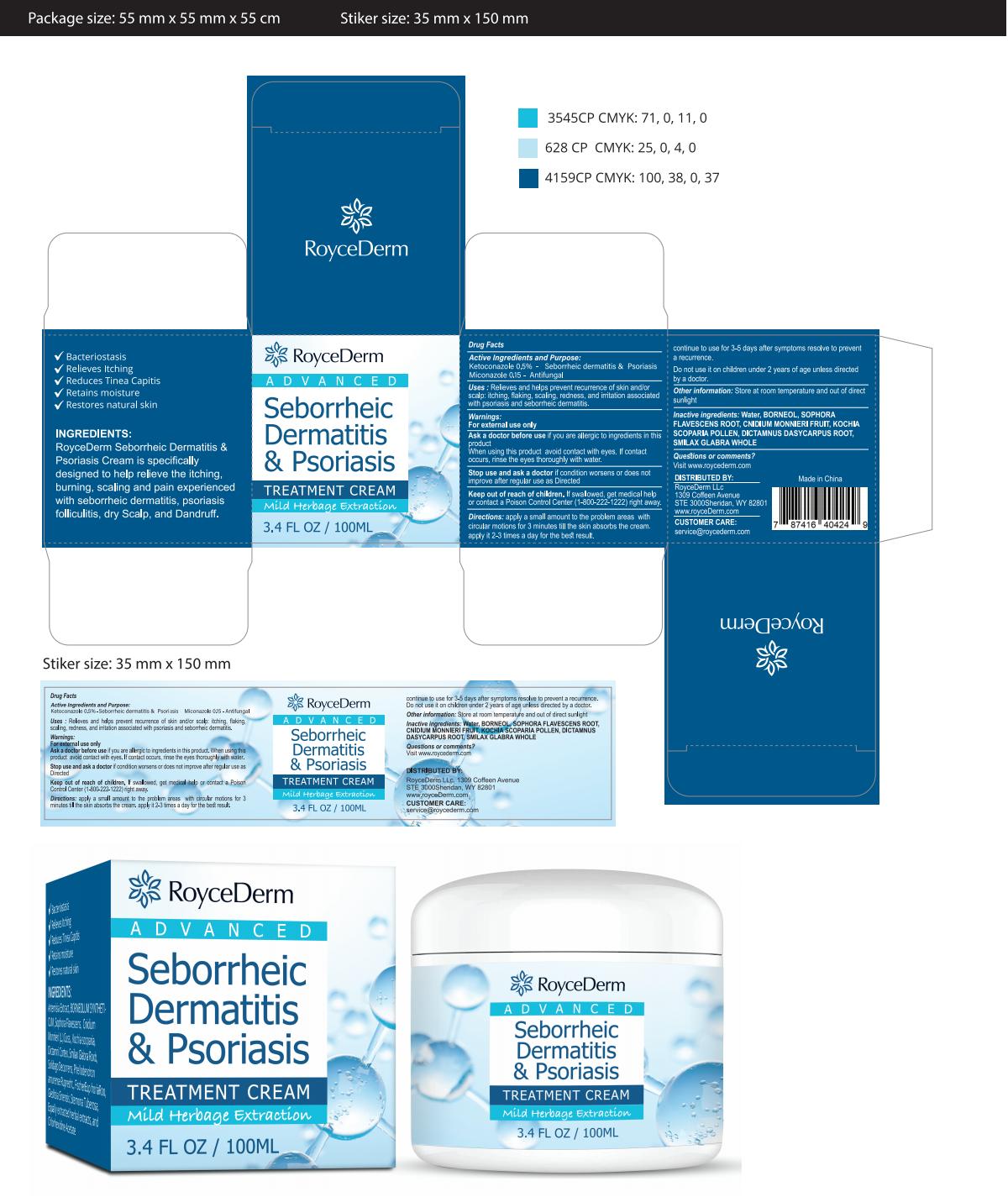
Label: ROYCEDERM SEBORRHEIC DERMATITIS PSORIASIS- dermatitis cream cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 83771-001-01 - Packager: Inner Mongolia Green source pharmaceutical Products Co., LTD
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 27, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Active IngredientKetoconazole 0.5% Miconazole 0.15
-
PurposeAntifungal - Seborrheic dermatitis & Psoriasis
-
UseRelieves and helps prevent recurrence of skin and/orscalp: itching, flaking, scaling, redness, and irritation associatedwith psoriasis and seborrheic dermatitis.
-
WarningsFor external use only
-
Do not useDo not use it on children under 2 years of age unless directedby a doctor.
-
Stop Useif condition worsens or does notimprove after regular use as Directed
-
Ask Doctorif you are allergic to ingredients in thisproductWhen using this product avoid contact with eyes. lf contactoccurs, rinse the eyes thoroughly with water.
-
Keep Oot Of Reach Of Childrenlf swallowed, get medical help or contact a Poison Control Center (1-800-222-1222) right away
-
Directionsapply a small amount to the problem areas withcircular motions for 3 minutes till the skin absorbs the cream.apply it 2-3 times a day for the best result - continue to use for 3-5 days after ...
-
Other informationStore at room temperature and out of direct sunlight
-
Inactive ingredientsWater, BORNEOL, SOPHORAFLAVESCENS ROOT, CNIDIUM MONNIERI FRUIT, KOCHIASCOPARIA POLLEN,DICTAMNUS DASYCARPUS ROOTSMILAX GLABRA WHOLE
-
QuestionsVisit www.roycederm.com
-
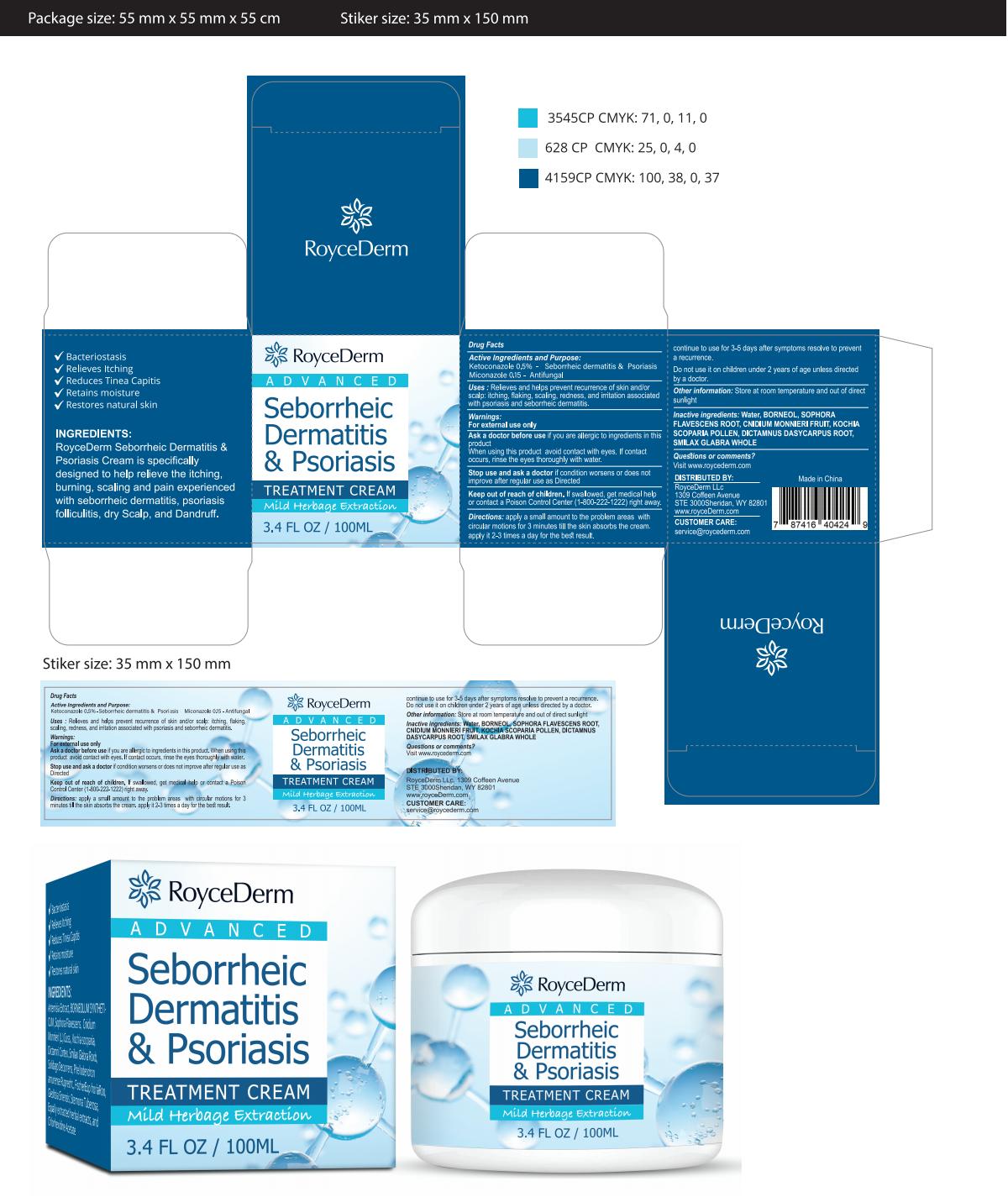
PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCEProduct Information