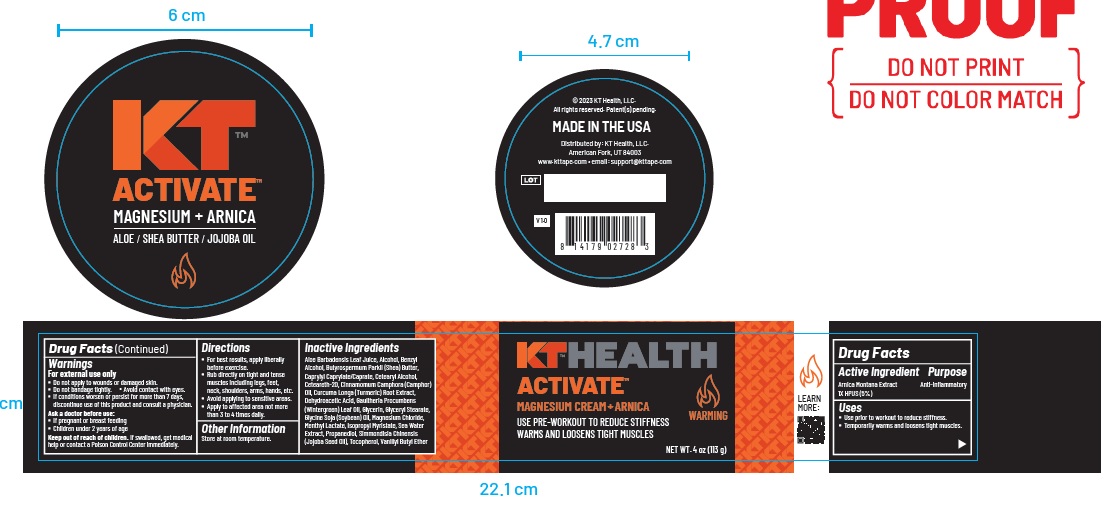
Label: KT ACTIVATE MAGNESIUM ARNICA- arnica montana whole cream
- NDC Code(s): 73044-105-01
- Packager: KT Health LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated November 18, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENTActive Ingredients Purpose - Arnica Montana Extract - 1X HPUS (5% ...
- PURPOSE
-
Uses
Use prior to workout to reduce stiffness. Temporarily warms and loosens tight muscles.
-
Warnings
For external use only - Do not apply to wounds or damaged skin. Do not bandage tightly. Avoid contact with eyes. If conditions worsen or persist for more than 7 days, discontinue use of this ...
- KEEP OUT OF REACH OF CHILDREN
-
Directions
For best results, apply liberally before exercise. Rub directly on tight and tense muscles including legs, feet, neck, shoulders, arms, hands, etc. Avoid applying to sensitive areas. Apply to ...
-
Inactive Ingredients
Aloe Barbadensis Leaf Juice, Alcohol, Benzyl Alcohol, Butyrospermum Parkii (Shea) Butter, Caprylyl Caprylate/Caprate, Cetearyl Alcohol, Ceteareth-20, Cinnamomum Camphora (Camphor) Oil ...
-
Other Information
Store at room temperature.
-
Product label
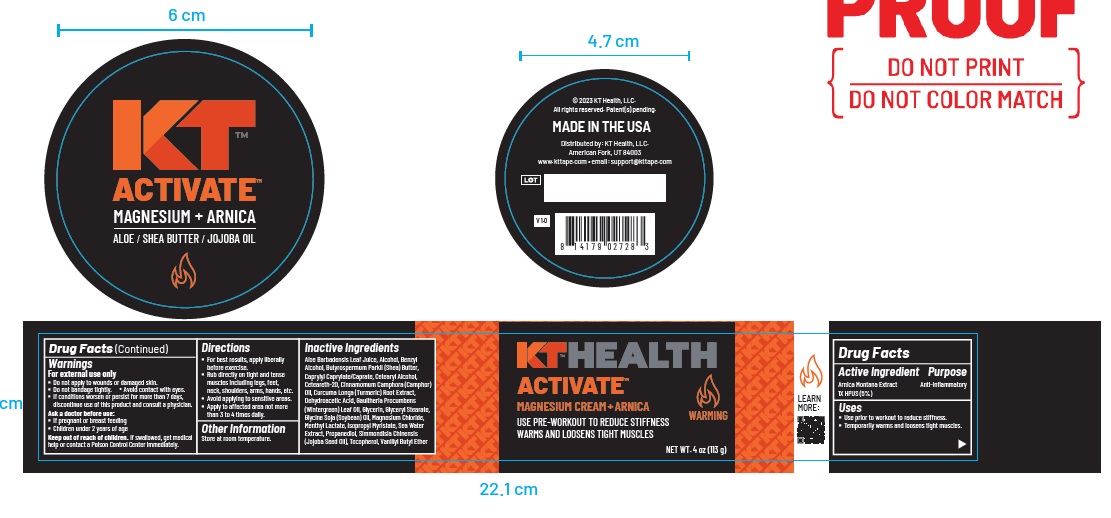
-
INGREDIENTS AND APPEARANCEProduct Information