Label: VOYDEYA- danicopan tablet, film coated
VOYDEYA- danicopan kit
- NDC Code(s): 25682-040-21, 25682-040-90, 25682-043-04, 25682-043-21, view more
- Packager: Alexion Pharmaceuticals Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated December 6, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use VOYDEYA safely and effectively. See full prescribing information for VOYDEYA. VOYDEYA™ (danicopan) tablets, for oral use ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: SERIOUS INFECTIONS CAUSED BY ENCAPSULATED BACTERIA
VOYDEYA, a complement inhibitor, increases the risk of serious infections, especially those caused by encapsulated bacteria, such as Neisseria meningitidis, Streptococcus pneumoniae, and Haemophilus influenzae type B [see Warnings and Precautions (5.1)]. Life-threatening and fatal infections with encapsulated bacteria have occurred in patients treated with complement inhibitors. These infections may become rapidly life-threatening or fatal if not recognized and treated early.
- Complete or update vaccination for encapsulated bacteria specifically, Neisseria meningitidis and Streptococcus pneumoniae at least 2 weeks prior to the first dose of VOYDEYA, unless the risks of delaying therapy with VOYDEYA outweigh the risk of developing a serious infection. Comply with the most current Advisory Committee on Immunization Practices (ACIP) recommendations for vaccinations against encapsulated bacteria in patients receiving a complement inhibitor. See Warnings and Precautions (5.1) for additional guidance on the management of the risk of serious infections caused by encapsulated bacteria.
- Patients receiving VOYDEYA are at increased risk for invasive disease caused by encapsulated bacteria, even if they develop antibodies following vaccination. Monitor patients for early signs and symptoms of serious infections and evaluate immediately if infection is suspected.
Because of the risk of serious infections caused by encapsulated bacteria, VOYDEYA is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the VOYDEYA REMS [see Warnings and Precautions (5.2)].
Close -
1 INDICATIONS AND USAGEVOYDEYA is indicated as add-on therapy to ravulizumab or eculizumab for the treatment of extravascular hemolysis (EVH) in adults with paroxysmal nocturnal hemoglobinuria (PNH). Limitations of ...
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Vaccination and Prophylaxis for Encapsulated Bacterial Infections - Vaccinate patients against encapsulated bacteria, including Neisseria meningitidis (serogroups A, C, W, Y, and ...
-
3 DOSAGE FORMS AND STRENGTHSTablets: 50 mg, white to off-white, round, film-coated, printed with "DCN" above "50" debossed on one side, plain on the other side. 100 mg, white to off-white, round film-coated, printed with ...
-
4 CONTRAINDICATIONSVOYDEYA is contraindicated for initiation in patients with unresolved serious infection caused by encapsulated bacteria, including Neisseria meningitidis, Streptococcus pneumoniae, or Haemophilus ...
-
5 WARNINGS AND PRECAUTIONS5.1 Serious Infections Caused by Encapsulated Bacteria - VOYDEYA, a complement inhibitor, increases a patient's susceptibility to serious, life-threatening, or fatal infections caused by ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are discussed in greater detail in other sections of the labeling: Serious Infections Caused by Encapsulated Bacteria [see Warnings and ...
-
7 DRUG INTERACTIONS7.1 BCRP Substrates - Danicopan is a Breast Cancer Resistance Protein (BCRP) inhibitor. Concomitant use of VOYDEYA with a BCRP substrate increases the plasma concentrations of the BCRP substrate ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no available data on VOYDEYA use in pregnant individuals to evaluate for a drug-associated risk of major birth defects, miscarriage or adverse maternal ...
-
10 OVERDOSAGESerum ALT elevations occurred after treatment cessation without a taper in 2 healthy subjects who received danicopan 500 mg and 800 mg twice a day. These abnormal ALT findings were transient, with ...
-
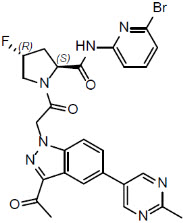
11 DESCRIPTIONDanicopan is a small molecule complement Factor D inhibitor. Its chemical name is (2S,4R)-1-{[3-acetyl-5-(2-methylpyrimidin-5-yl)-1H-indazol-1-yl ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Danicopan binds reversibly to complement Factor D and selectively inhibits the alternative complement pathway. Danicopan prevents the cleavage of complement Factor B ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Danicopan was not carcinogenic in the 6-month carcinogenicity study in the TgRasH2 mouse model. Danicopan was not ...
-
14 CLINICAL STUDIESParoxysmal Nocturnal Hemoglobinuria (PNH) The efficacy of VOYDEYA in adults with PNH and clinically significant EVH was assessed in a multiple-region, randomized, double-blind ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGVOYDEYA (danicopan) tablets are available in the doses and packages listed in Table 4. Table 4 VOYDEYA Tablet Presentations - DoseTablet StrengthFilm-Coated Tablet MarkingsTablet ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Medication Guide). Serious Infections Caused by Encapsulated Bacteria - Advise patients of the risk of serious infection. Inform ...
-
SPL UNCLASSIFIED SECTIONManufactured for: Alexion Pharmaceuticals, Inc. 121 Seaport Boulevard - Boston, MA 02210 USA - This product, or its use, may be covered by one or more US patents, including US Patent No. 9,796,741 ...
-
MEDICATION GUIDEMEDICATION GUIDE - VOYDEYA™(voi-day-uh) (danicopan) tablets, for oral use - This Medication Guide has been approved by the U.S. Food and Drug Administration. Issued ...
-
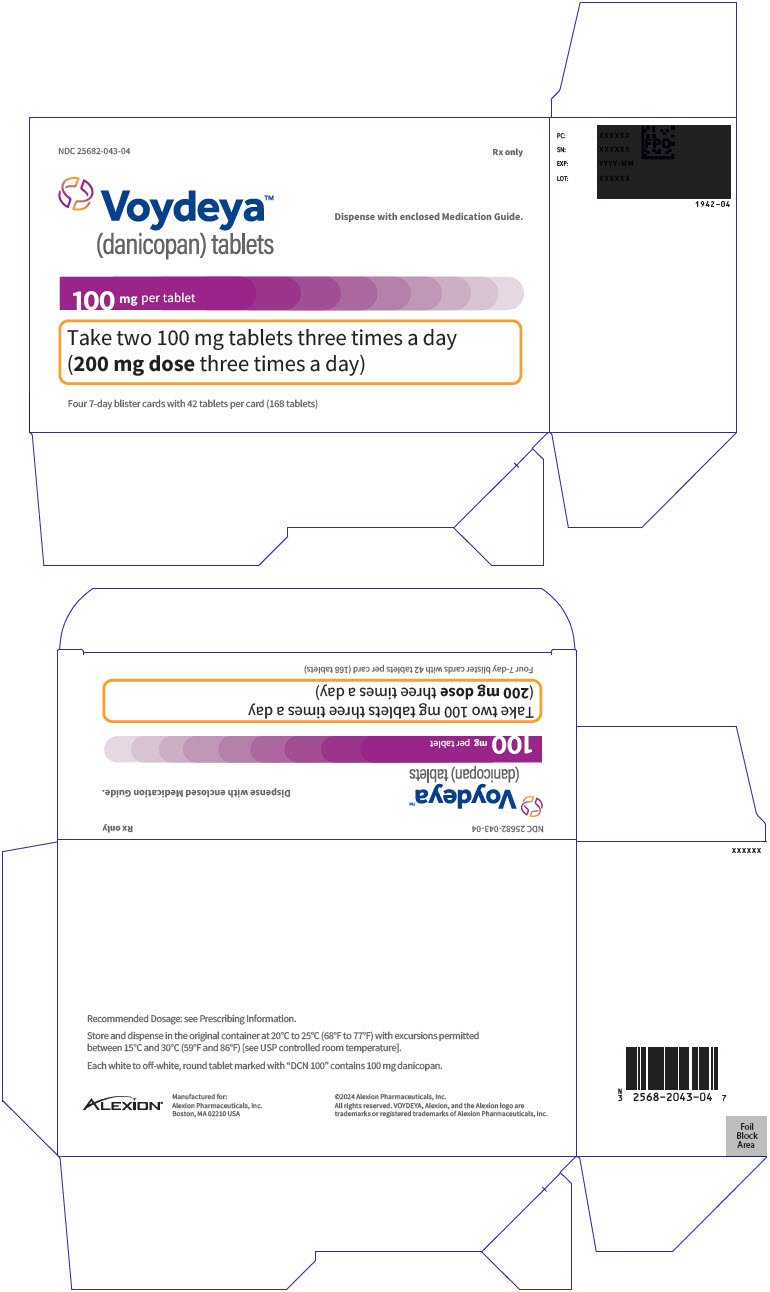
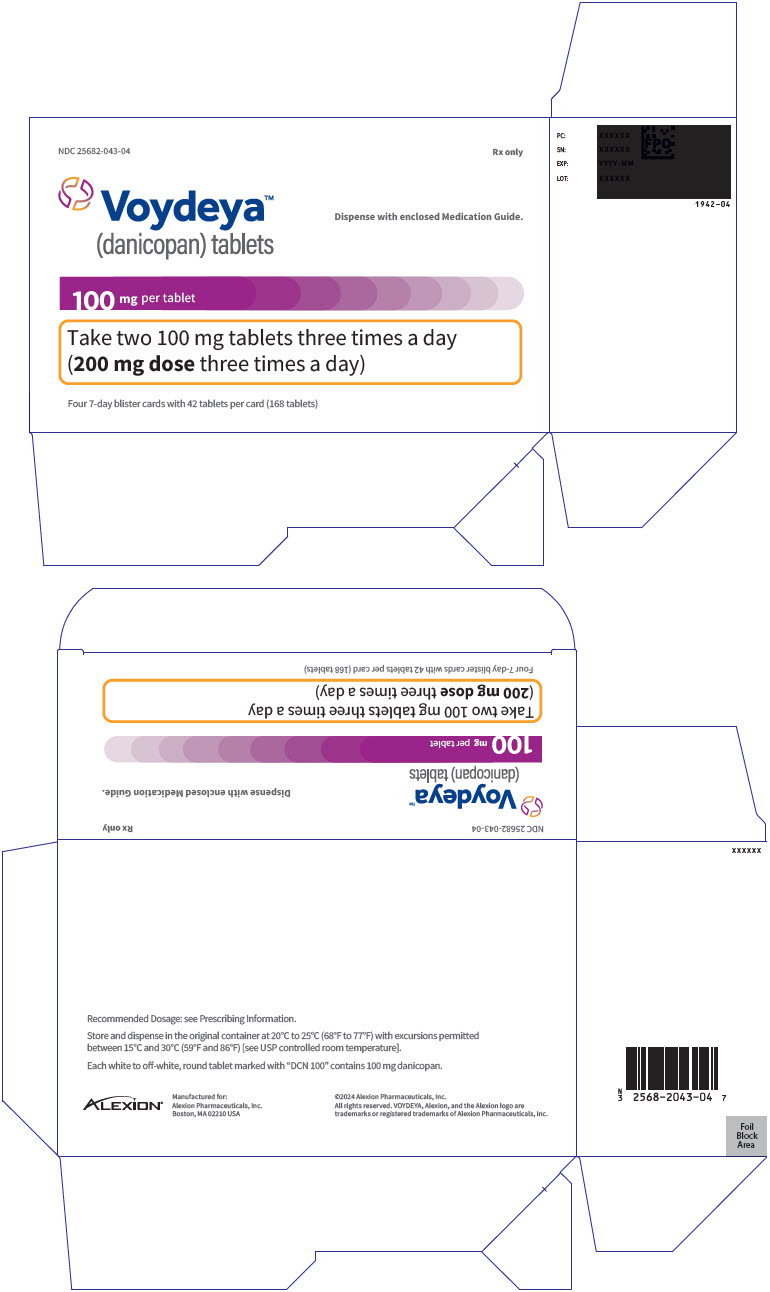
PRINCIPAL DISPLAY PANEL - 100 mg Tablet Blister Pack Carton - 043-04NDC 25682-043-04 - Rx only - Voydeya™ (danicopan) tablets - Dispense with enclosed Medication Guide. 100 mg per tablet - Take two 100 mg tablets three times a day - (200 mg dose three times a day) Four ...
-
PRINCIPAL DISPLAY PANEL - 100 mg Tablet Bottle Carton - 043-92NDC 25682-043-92 - Rx only - Voydeya™ (danicopan) tablets - Dispense with enclosed Medication Guide. 100 mg - 180 tablets - 90 x 100 mg tablets per bottle
-
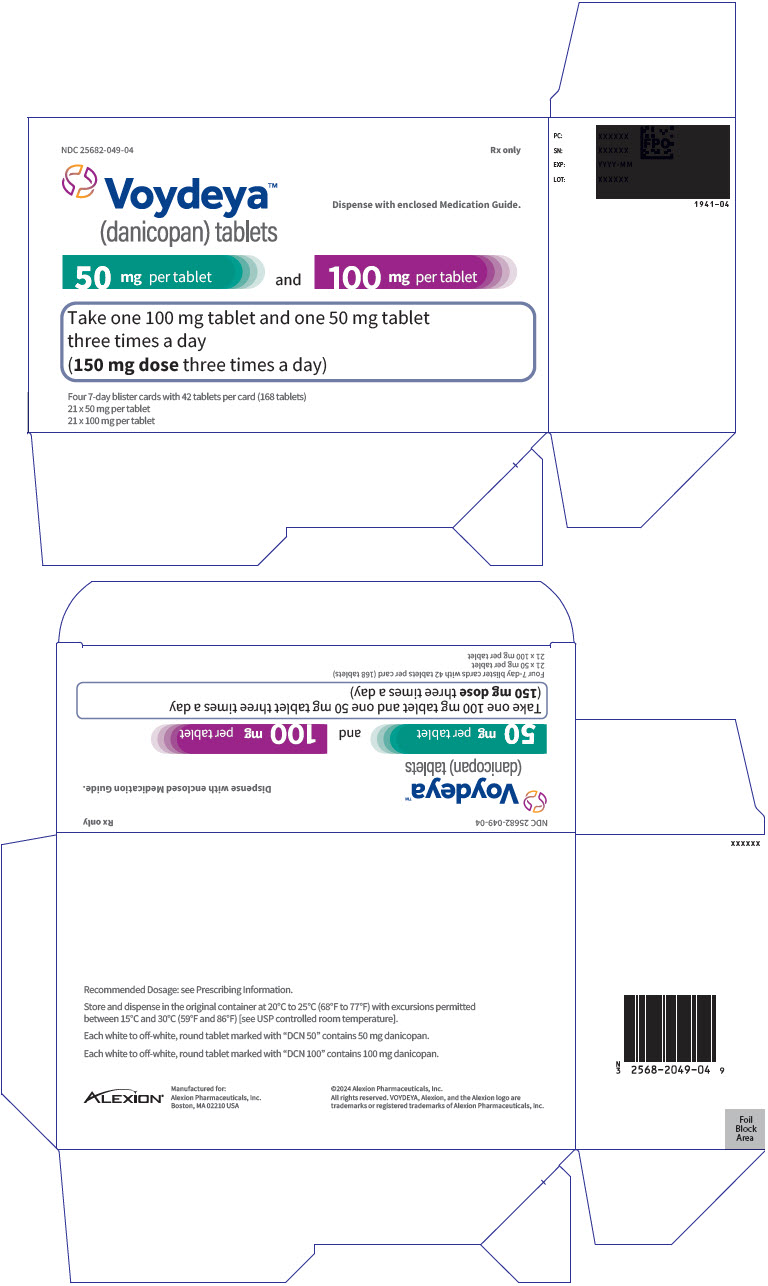
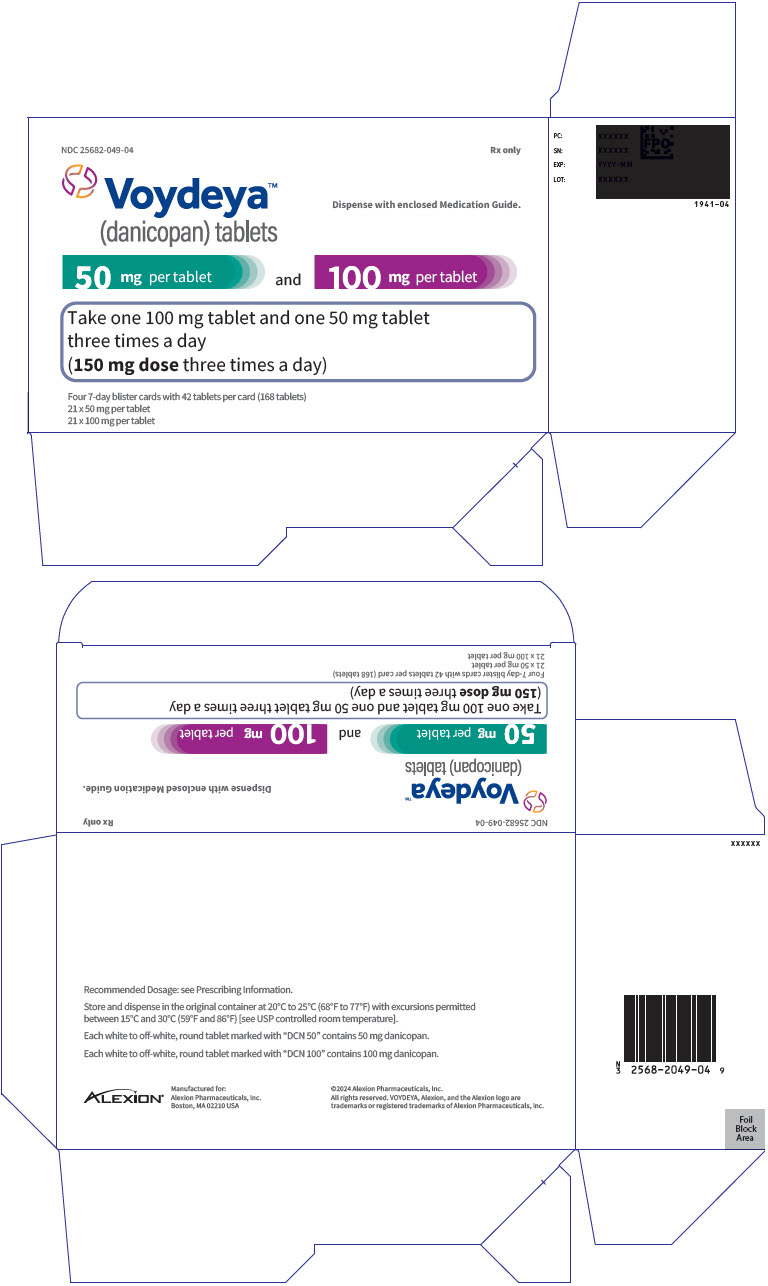
PRINCIPAL DISPLAY PANEL - Kit Carton - 049NDC 25682-049-04 - Rx only - Voydeya™ (danicopan) tablets - Dispense with enclosed Medication Guide. 50 mg per tablet - and - 100 mg per tablet - Take one 100 mg tablet and one 50 mg tablet - three times ...
-
PRINCIPAL DISPLAY PANEL - Kit Carton - 046NDC 25682-046-92 - Rx only - Voydeya™ (danicopan) tablets - Dispense with enclosed Medication Guide. 50 mg - and - 100 mg - Take one 100 mg tablet and one 50 mg tablet - three times a day - (150 mg dose ...
-
INGREDIENTS AND APPEARANCEProduct Information