Label: REZZAYO- rezafungin injection, powder, lyophilized, for solution
- NDC Code(s): 70842-240-01
- Packager: Melinta Therapeutics, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated October 14, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use REZZAYO safely and effectively. See full prescribing information for REZZAYO. REZZAYO™ (rezafungin for injection), for intravenous ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE1.1 Indication - REZZAYO is indicated in patients 18 years of age or older who have limited or no alternative options for the treatment of candidemia and invasive candidiasis [see Microbiology ...
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosage - Administer the recommended dosage of REZZAYO once weekly by intravenous (IV) infusion, with an initial 400 mg loading dose, followed by a 200 mg dose once weekly ...
-
3 DOSAGE FORMS AND STRENGTHSFor injection: 200 mg of rezafungin as a sterile white to pale yellow solid (cake or powder) for reconstitution in a single-dose glass vial.
-
4 CONTRAINDICATIONSREZZAYO is contraindicated in patients with known hypersensitivity to rezafungin or other echinocandins.
-
5 WARNINGS AND PRECAUTIONS5.1 Infusion-Related Reactions - Infusion-related reactions, including flushing, sensation of warmth, urticaria, nausea, and chest tightness have been observed in clinical trials with REZZAYO. If ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described elsewhere in the labeling: Infusion-related Reactions [see Warnings and Precautions (5.1)] Hepatic Adverse Reactions [see ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no data on the use of REZZAYO during pregnancy to evaluate for a drug-associated risk of major birth defects, miscarriage, or other adverse maternal or ...
-
10 OVERDOSAGENo cases of overdose were reported during the clinical studies. Rezafungin is highly protein bound and not anticipated to be dialyzable.
-
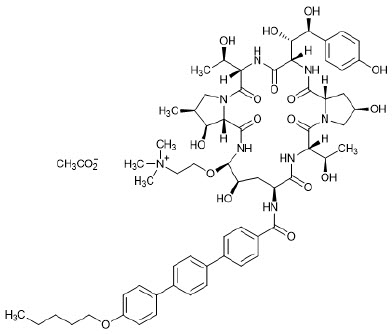
11 DESCRIPTIONREZZAYO (rezafungin for injection), for intravenous use is a sterile solid (cake or powder) that contains rezafungin acetate. Rezafungin acetate is a semisynthetic lipopeptide synthesized from a ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Rezafungin is an echinocandin antifungal drug [see Microbiology (12.4)]. 12.2 Pharmacodynamics - Rezafungin exposures achieved with the recommended dosage regimen ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Carcinogenicity studies with rezafungin have not been conducted. Mutagenesis - Rezafungin was negative in a standard ...
-
14 CLINICAL STUDIESThe safety and efficacy of REZZAYO in the treatment of patients with candidemia and/or invasive candidiasis (IC) were evaluated in a multicenter, randomized, double-blind study (Trial 1 ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - REZZAYO (rezafungin for injection) is supplied as sterile white to pale yellow solid (cake or powder) in a single-dose 20 mL Type I glass vial with a stopper, an aluminum ...
-
17 PATIENT COUNSELING INFORMATIONPhotosensitivity - Advise patients to use protection against sun exposure and other sources of UV radiation during treatment because REZZAYO may cause photosensitivity [see Warnings and ...
-
PRINCIPAL DISPLAY PANELCarton Label - One 200 mg Sterile Single-dose Vial - REZZAYO - PRINCIPAL DISPLAY PANEL - NDC 70842-240-01 - Rx only - REZZAYO™ (rezafungin for injection) 200 mg per vial - *Vial contains ...
-
INGREDIENTS AND APPEARANCEProduct Information