Label: ZURZUVAE- zuranolone capsule
- NDC Code(s): 64406-029-01, 64406-030-01, 64406-030-02, 64406-031-01
- Packager: Biogen MA Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CIV
- Marketing Status: New Drug Application
Drug Label Information
Updated November 5, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ZURZUVAE safely and effectively. See full prescribing information for ZURZUVAE. ZURZUVAETM (zuranolone) capsules, for oral use ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: IMPAIRED ABILITY TO DRIVE OR ENGAGE IN OTHER POTENTIALLY HAZARDOUS ACTIVITIES
ZURZUVAE causes driving impairment due to central nervous system (CNS) depressant effects [see Warnings and Precautions (5.1, 5.2)].
Advise patients not to drive or engage in other potentially hazardous activities until at least 12 hours after ZURZUVAE administration for the duration of the 14-day treatment course. Inform patients that they may not be able to assess their own driving competence, or the degree of driving impairment caused by ZURZUVAE [see Warnings and Precautions (5.1)].
Close -
1 INDICATIONS AND USAGE
ZURZUVAE is indicated for the treatment of postpartum depression (PPD) in adults.
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage - The recommended dosage of ZURZUVAE is 50 mg taken orally once daily in the evening for 14 days. Administer ZURZUVAE with fat-containing food (e.g., 400 to 1,000 ...
-
3 DOSAGE FORMS AND STRENGTHS
Capsules: 20 mg: light-orange cap, ivory to light-yellow body, printed with “S-217 20 mg” on body of capsule in black - 25 mg: light-orange cap, light-orange body, printed with “S-217 25 mg” on ...
-
4 CONTRAINDICATIONS
None.
-
5 WARNINGS AND PRECAUTIONS
5.1 Impaired Ability to Drive or Engage in Other Potentially Hazardous Activities - ZURZUVAE causes driving impairment due to central nervous system (CNS) depressant effects [see Warnings and ...
-
6 ADVERSE REACTIONS
The following adverse reactions are discussed in more detail in other sections of the labeling: Impaired Ability to Drive or Engage in Other Potentially Hazardous Activities [see Warnings and ...
-
7 DRUG INTERACTIONS
Table 4 displays clinically important drug interactions with ZURZUVAE. Table 4 Clinically Important Drug Interactions with ZURZUVAE - CNS Depressant Drugs and Alcohol - Clinical ImpactDue ...
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy - Pregnancy Exposure Registry - There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to antidepressants, including ZURZUVAE, during ...
-
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance - ZURZUVAE contains zuranolone, a Schedule IV controlled substance. 9.2 Abuse - Zuranolone has abuse potential with associated risks of misuse, abuse, and substance ...
-
10 OVERDOSAGE
There was a case of intentional overdose with ZURZUVAE reported during premarketing clinical trials. The patient took 330 mg (6.5 times the maximum recommended dose) of ZURZUVAE and was reported ...
-
11 DESCRIPTION
ZURZUVAE contains zuranolone, a neuroactive steroid gamma-aminobutyric acid (GABA) A receptor positive modulator. The chemical name is 1-[(3α, 5β)-3-hydroxy-3-methyl-20 ...
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action - The mechanism of action of zuranolone in the treatment of PPD is not fully understood, but is thought to be related to its positive allosteric modulation of GABAA ...
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Oral administration of zuranolone in a 26-week carcinogenicity study in transgenic mice (0, 10, 30, or 100 ...
-
14 CLINICAL STUDIES
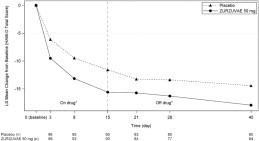
14.1 Postpartum Depression - The efficacy of ZURZUVAE for the treatment of postpartum depression (PPD) in adults was demonstrated in two randomized, placebo-controlled, double-blind, multicenter ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied - ZURZUVAE (zuranolone) is supplied as 20 mg, 25 mg, and 30 mg capsules as follows: Capsule - Strength - Capsule Colors - Capsule Markings - Packaging Configuration - NDC - 20 ...
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide). Impaired Ability to Drive or Engage in Other Potentially Hazardous Activities - Inform patients that ZURZUVAE ...
-
MEDICATION GUIDEThis Medication Guide has been approved by the U.S. Food and Drug Administration. Revised: 07/2024 - MEDICATION GUIDE - ZURZUVAE™ (zur-ZOO-vay) (zuranolone) capsules, for oral use ...
-
PRINCIPAL DISPLAY PANELPRINCIPAL DISPLAY PANEL - 30 mg Bottle Carton - NDC 64406-031-01 - Rx Only - ZURZUVAE™ CIV - (zuranolone) capsules - 30 mg - For oral use - Attention: Dispense - with accompanying - Medication Guide. 14 ...
-
PRINCIPAL DISPLAY PANELPRINCIPAL DISPLAY PANEL - 25 mg Blister Pack Carton - ZURZUVAE™ CIV - (zuranolone) capsules - 25 mg per capsule - For oral use - Attention: Dispense - with accompanying - Medication Guide. 28 Capsules - NDC ...
-
PRINCIPAL DISPLAY PANELPRINCIPAL DISPLAY PANEL - 20 mg Bottle Carton - NDC 64406-029-01 - Rx Only - ZURZUVAE™ CIV - (zuranolone) capsules - 20 mg - For oral use - Attention: Dispense - with accompanying - Medication Guide. 14 ...
-
INGREDIENTS AND APPEARANCEProduct Information