Label: CUVRIOR- trientine tetrahydrochloride tablet, film coated
- NDC Code(s): 81802-001-08, 81802-001-72
- Packager: Orphalan SA
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated June 19, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use CUVRIOR safely and effectively. See full prescribing information for CUVRIOR. CUVRIOR® (trientine tetrahydrochloride) tablets, for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGECUVRIOR is indicated for the treatment of adult patients with stable Wilson's disease who are de-coppered and tolerant to penicillamine.
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosage and Administration - The recommended starting total daily dosage of CUVRIOR in adult patients is 300 mg up to 3,000 mg taken orally in divided doses (two times daily) ...
-
3 DOSAGE FORMS AND STRENGTHSTablets: 300 mg of trientine tetrahydrochloride (equivalent to 150 mg of trientine), oblong, yellow coated, functionally scored, printed with OL75 on each side of score line in black ink. Each ...
-
4 CONTRAINDICATIONSCUVRIOR is contraindicated in patients with hypersensitivity to trientine or to any of the excipients in CUVRIOR [see Warnings and Precautions (5.4)].
-
5 WARNINGS AND PRECAUTIONS5.1 Potential for Worsening of Clinical Symptoms at Initiation of Therapy - Worsening of clinical symptoms, including neurological deterioration, may occur at the beginning of CUVRIOR therapy due ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described elsewhere in the labeling: Potential for Worsening of Clinical Symptoms at Initiation of Therapy [see Warnings and Precautions ...
-
7 DRUG INTERACTIONS7.1 Mineral Supplements and Other Oral Drugs - CUVRIOR has the potential to chelate non-copper cations in mineral supplements and other oral drugs, and could be rendered ineffective prior to ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Available data from published literature and postmarketing experience over several decades with use of trientine for the treatment of Wilson's disease have not ...
-
10 OVERDOSAGEOccasional cases of trientine overdose have been reported. A large overdose of 60 g of trientine hydrochloride (equivalent to 80 g CUVRIOR) resulted in nausea, vomiting, dizziness, mild acute ...
-
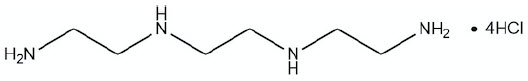
11 DESCRIPTIONCUVRIOR contains trientine tetrahydrochloride which is a salt of trientine, a copper chelator. The structural formula of trientine tetrahydrochloride is: Molecular ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Trientine, a copper chelator, eliminates absorbed copper from the body by forming a stable complex that is then eliminated through urinary excretion. Trientine also ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Oral carcinogenicity studies have not been conducted with trientine. Mutagenesis - Trientine was positive in ...
-
14 CLINICAL STUDIESThe effectiveness of CUVRIOR for the treatment of adult patients with stable Wilson's disease who are decoppered and tolerant to penicillamine was demonstrated in a phase 3 trial (Trial 1). In ...
-
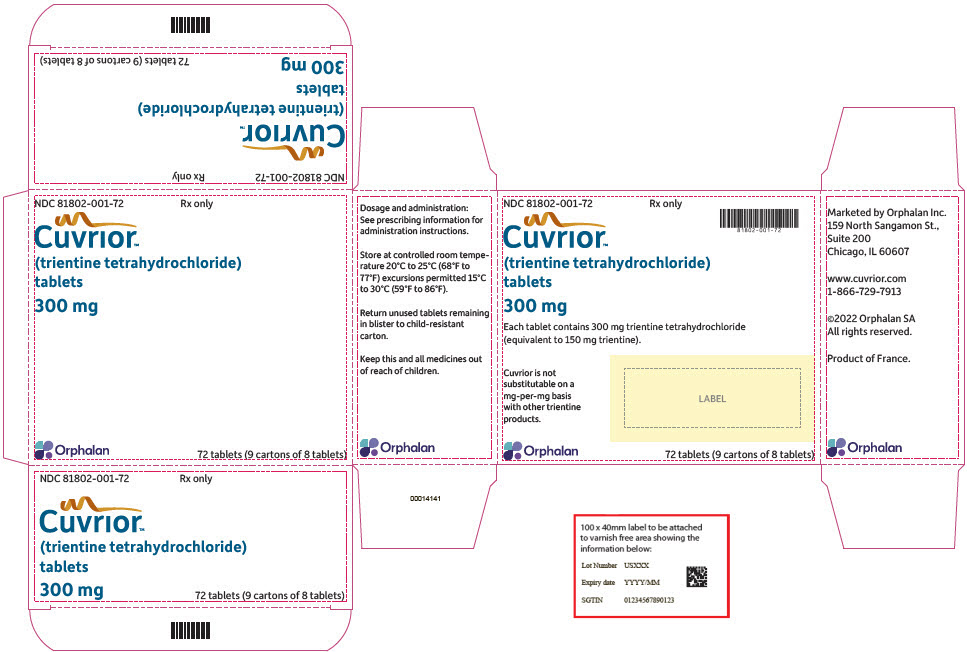
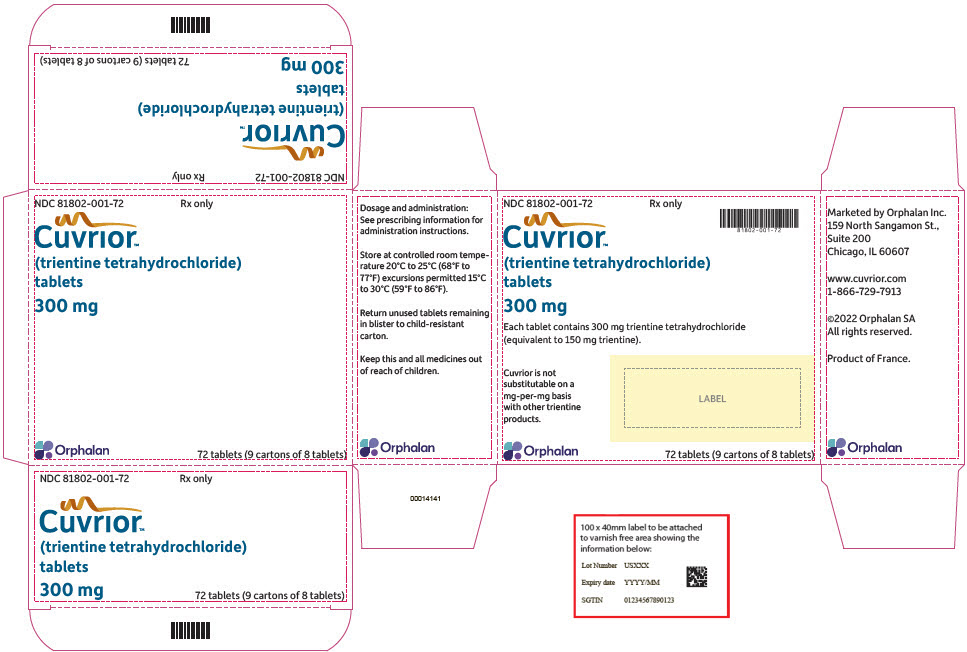
16 HOW SUPPLIED/STORAGE AND HANDLINGCUVRIOR tablets, 300 mg of trientine tetrahydrochloride, are oblong, yellow coated, functionally scored, and imprinted with OL75 on each side. Each large carton (NDC 81802-001-72) contains nine ...
-
17 PATIENT COUNSELING INFORMATIONAdministration Instructions - Advise patients or their caretaker(s) to [see Dosage and Administration (2.2)]: Take CUVRIOR on an empty stomach (at least 1 hour before meals or 2 hours after ...
-
SPL UNCLASSIFIED SECTIONMarketed by: Orphalan, Chicago, IL 60607 - Product of France - CUVRIOR is a registered trademark of Orphalan SA - ©2025 Orphalan SA, all rights reserved.
-
PRINCIPAL DISPLAY PANEL - 72 Tablet Blister Pack CartonNDC 81802-001-72 - Rx only - Cuvrior™ (trientine tetrahydrochloride) tablets - 300 mg - Orphalan - 72 tablets (9 cartons of 8 tablets)
-
INGREDIENTS AND APPEARANCEProduct Information