Label: BEQVEZ- fidanacogene elaparvovec-dzkt kit
- NDC Code(s): 0069-0422-01, 0069-2004-04, 0069-2004-14, 0069-2005-05, view more
- Packager: Pfizer Laboratories Div Pfizer Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated May 30, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use BEQVEZ safely and effectively. See full prescribing information for BEQVEZ. BEQVEZTM (fidanacogene elaparvovec-dzkt) injection ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE BEQVEZ is an adeno-associated virus vector-based gene therapy indicated for the treatment of adults with moderate to severe hemophilia B (congenital factor IX deficiency) who: • Currently use ...
-
2 DOSAGE AND ADMINISTRATION For one-time single-dose intravenous infusion only. Initiate and administer BEQVEZ in hospitals and other clinical centers under the supervision of a physician experienced in the treatment of ...
-
3 DOSAGE FORMS AND STRENGTHS BEQVEZ is supplied as a clear to slightly opalescent, colorless to slightly brown suspension for intravenous infusion with each mL containing 1 × 1013 vector genomes (vg). Each vial of BEQVEZ ...
-
4 CONTRAINDICATIONS None.
-
5 WARNINGS AND PRECAUTIONS 5.1 Hepatotoxicity - Intravenous administration of a liver-directed AAV vector could potentially lead to liver transaminase elevations. Transaminase elevations, particularly when observed in ...
-
6 ADVERSE REACTIONS The most common adverse reaction (incidence ≥5%) reported in clinical studies was an increase in transaminases. 6.1 Clinical Trials Experience - Because clinical trials are conducted under ...
-
7 DRUG INTERACTIONS No interaction studies have been performed. The use of BEQVEZ in patients receiving hepatotoxic medication or using hepatotoxic substances is limited. Use of hepatotoxic medications or substances ...
-
8 USE IN SPECIFIC POPULATIONS 8.1 Pregnancy - Risk Summary - BEQVEZ is not intended for administration in women. There are no data from the use of BEQVEZ in pregnant women. No animal reproductive studies have been ...
-
11 DESCRIPTION BEQVEZ (fidanacogene elaparvovec-dzkt) is an adeno-associated virus (AAV)-based gene therapy for intravenous infusion. BEQVEZ is based on recombinant DNA technology that consists of a recombinant ...
-
12 CLINICAL PHARMACOLOGY 12.1 Mechanism of Action - BEQVEZ (fidanacogene elaparvovec-dzkt) is a gene therapy designed to introduce in the transduced cells a functional copy of the factor IX gene encoding a ...
-
13 NONCLINICAL TOXICOLOGY 13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenicity, mutagenicity, reproductive toxicity, and impairment of fertility studies have not been conducted with BEQVEZ. In a ...
-
14 CLINICAL STUDIES The efficacy of BEQVEZ was evaluated in clinical study 1 (NCT03861273) which is an ongoing, prospective, open-label, single-arm, multi-national study. The study enrolled 45 adult male patients ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING 16.1 How Supplied - BEQVEZ is supplied as a clear to slightly opalescent, colorless to slightly brown suspension with each mL containing 1 × 1013 vg. BEQVEZ is shipped frozen (−100 °C to ...
-
17 PATIENT COUNSELING INFORMATION Inform patients that: • Pre-infusion blood tests will be necessary to look for factor IX inhibitors and detect pre-existing antibodies to AAVRh74var. If these tests are positive the patient will ...
-
PRINCIPAL DISPLAY PANEL – 1 mL Vial Label – NDC 0069-0422-01 NDC 0069-0422-01 - Rx only - fidanacogene - elaparvovec-dzkt - BEQVEZ™ 1 x 1013 vg/mL - Injection for Intravenous Use Only - Single-dose - Contains no preservative. Discard unused portion - DO NOT SHAKE. DO NOT ...
-
PRINCIPAL DISPLAY PANEL – Intermediate Carton Pfizer - fidanacogene elaparvovec-dzkt - BEQVEZ™ 1 x 1013 vector genomes (vg)/mL - Injection for Intravenous Use Only - Single-dose vials - NOT FOR INDIVIDUAL RESALE - Rx only
-
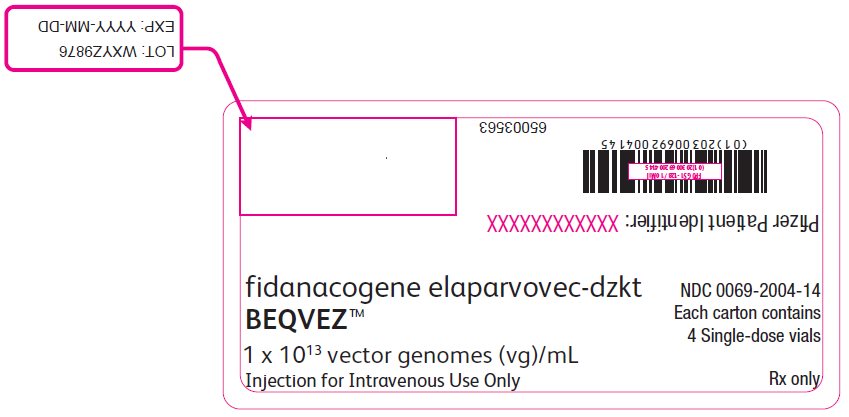
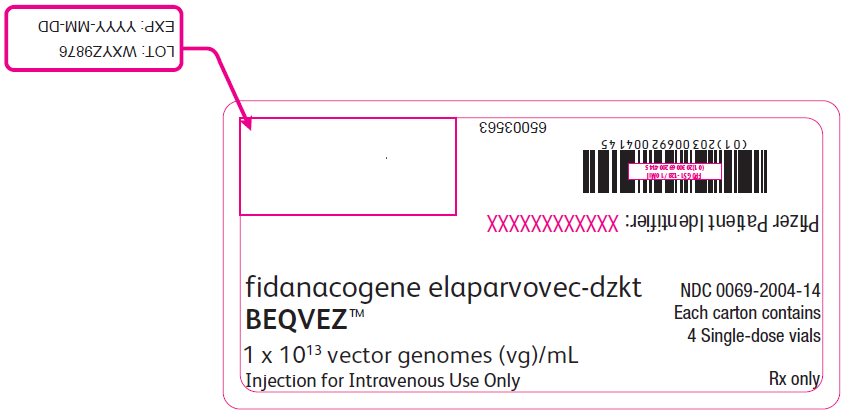
PRINCIPAL DISPLAY PANEL – Intermediate Carton Sticker – 4 single dose vials fidanacogene elaparvovec-dzkt - BEQVEZ™ 1 x 1013 vector genomes (vg)/mL - Injection for Intravenous Use Only - NDC 0069-2004-14 - Each carton contains - 4 Single-dose vials - Rx only
-
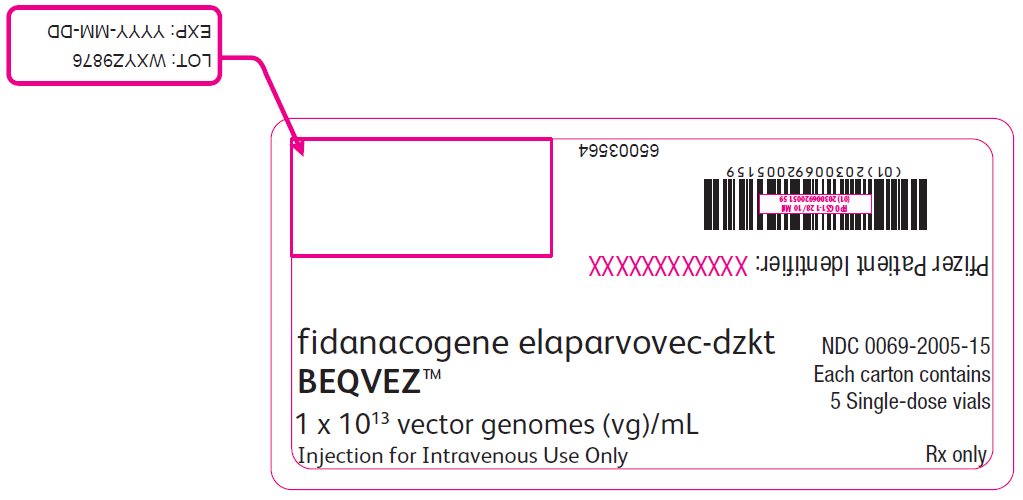
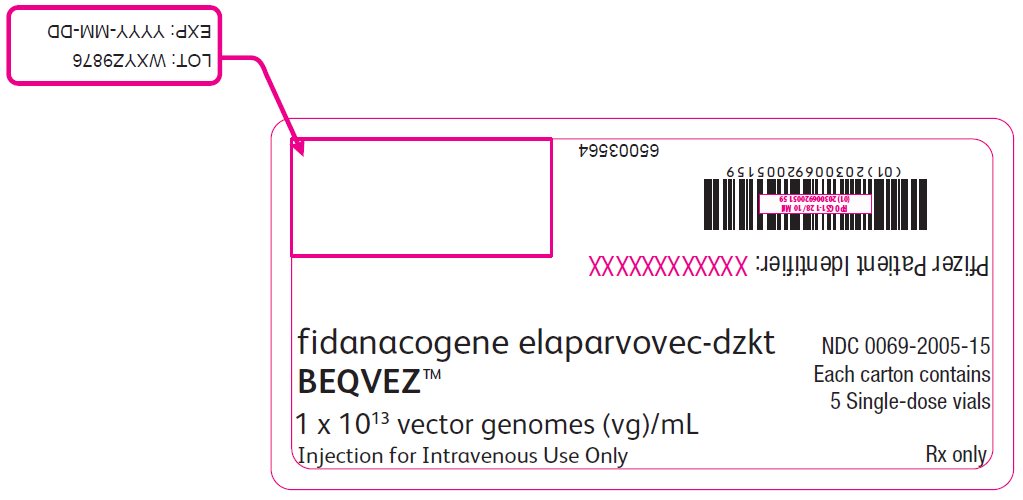
PRINCIPAL DISPLAY PANEL – Intermediate Carton Sticker – 5 single dose vials fidanacogene elaparvovec-dzkt - BEQVEZ™ 1 x 1013 vector genomes (vg)/mL - Injection for Intravenous Use Only - NDC 0069-2005-15 - Each carton contains - 5 Single-dose vials - Rx only
-
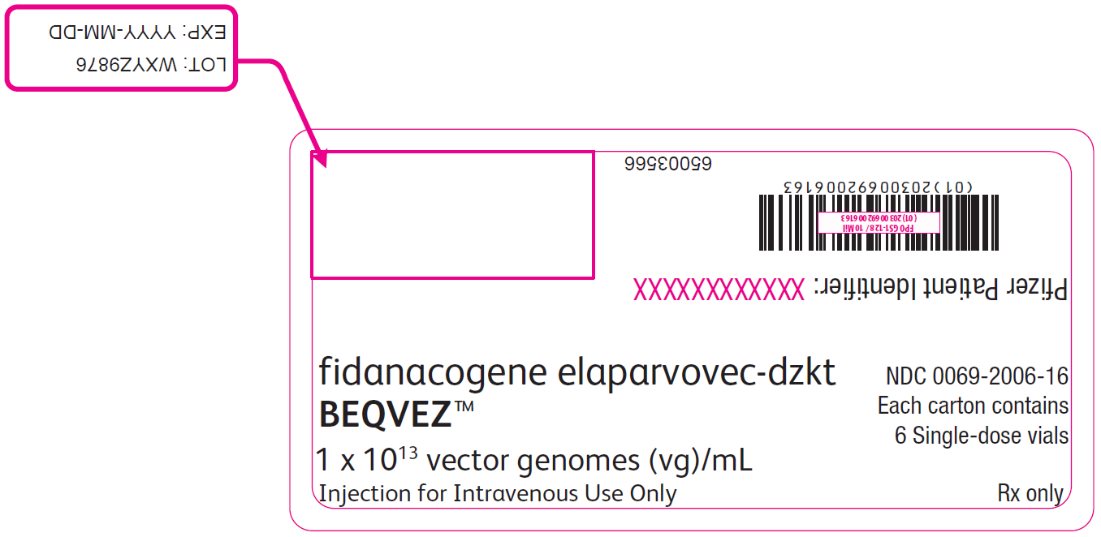
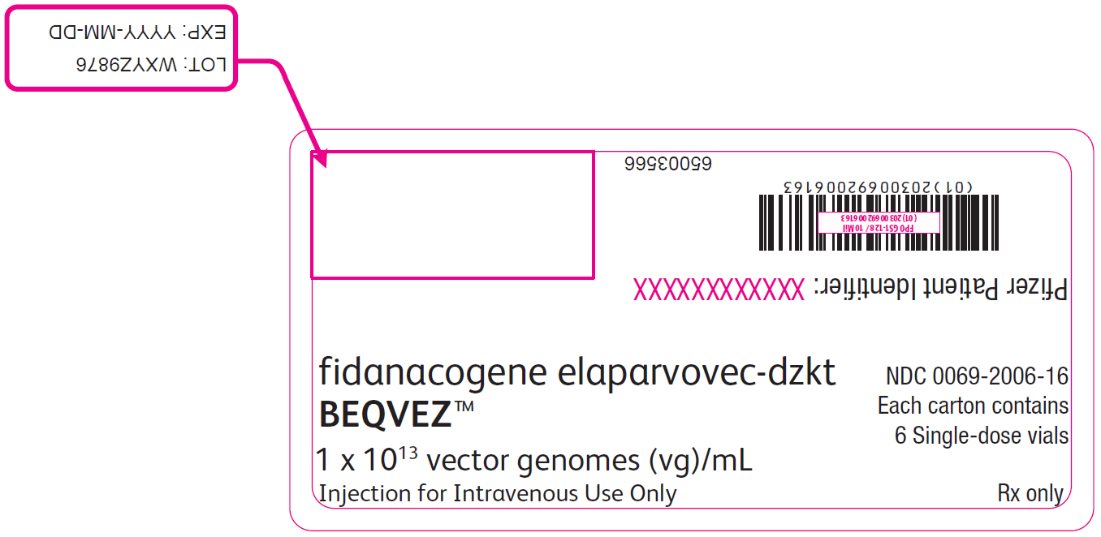
PRINCIPAL DISPLAY PANEL – Intermediate Carton Sticker – 6 single dose vials fidanacogene elaparvovec-dzkt - BEQVEZ™ 1 x 1013 vector genomes (vg)/mL - Injection for Intravenous Use Only - NDC 0069-2006-16 - Each carton contains - 6 Single-dose vials - Rx only
-
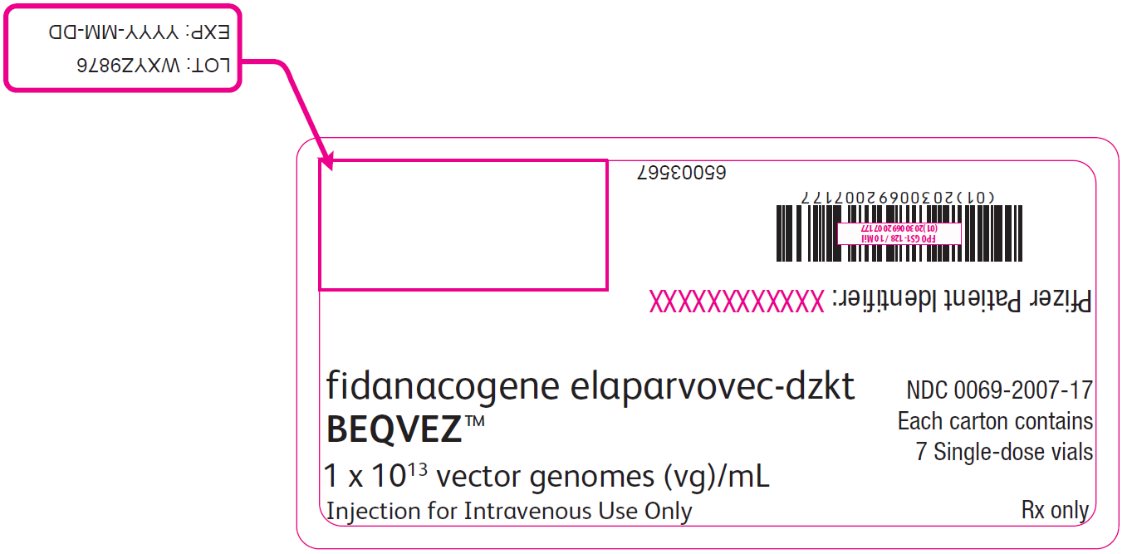
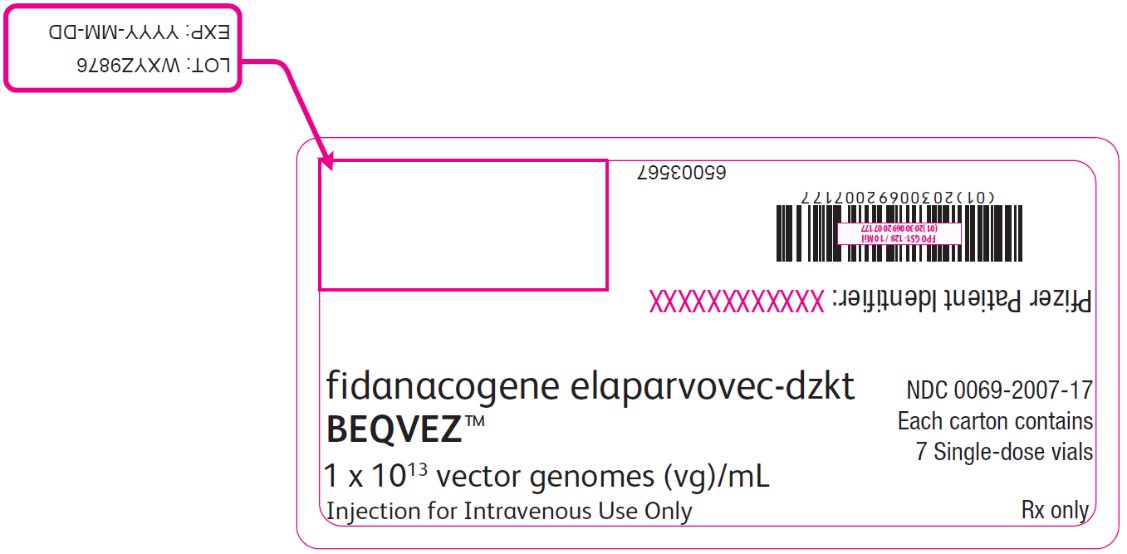
PRINCIPAL DISPLAY PANEL – Intermediate Carton Sticker – 7 single dose vials fidanacogene elaparvovec-dzkt - BEQVEZ™ 1 x 1013 vector genomes (vg)/mL - Injection for Intravenous Use Only - NDC 0069-2007-17 - Each carton contains - 7 Single-dose vials - Rx only
-
PRINCIPAL DISPLAY PANEL – Outer Carton Pfizer - fidanacogene elaparvovec-dzkt - BEQVEZ™ 1 x 1013 vector genomes (vg)/mL - Injection for Intravenous Use Only - Single-dose vials - Rx only
-
PRINCIPAL DISPLAY PANEL – Outer Carton Sticker – NDC 0069-2004-04 NDC 0069-2004-04 - (Contains 1 carton of 4 Single-dose vials) fidanacogene elaparvovec-dzkt - BEQVEZ™ 1 x 1013 vector genomes (vg)/mL - Injection for Intravenous Use Only - STORAGE: BEQVEZ™ is shipped and ...
-
PRINCIPAL DISPLAY PANEL – Outer Carton Sticker – NDC 0069-2005-05 NDC 0069-2005-05 - (Contains 1 carton of 5 Single-dose vials) fidanacogene elaparvovec-dzkt - BEQVEZ™ 1 x 1013 vector genomes (vg)/mL - Injection for Intravenous Use Only - STORAGE: BEQVEZ™ is shipped and ...
-
PRINCIPAL DISPLAY PANEL – Outer Carton Sticker – NDC 0069-2006-06 NDC 0069-2006-06 - (Contains 1 carton of 6 Single-dose vials) fidanacogene elaparvovec-dzkt - BEQVEZ™ 1 x 1013 vector genomes (vg)/mL - Injection for Intravenous Use Only - STORAGE: BEQVEZ™ is shipped and ...
-
PRINCIPAL DISPLAY PANEL – Outer Carton Sticker – NDC 0069-2007-07 NDC 0069-2007-07 - (Contains 1 carton of 7 Single-dose vials) fidanacogene elaparvovec-dzkt - BEQVEZ™ 1 x 1013 vector genomes (vg)/mL - Injection for Intravenous Use Only - STORAGE: BEQVEZ™ is shipped and ...
-
INGREDIENTS AND APPEARANCEProduct Information