Label: REGENIVADE HAIR GROWTH FOAM (regenivade hair growth- serum foam liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 81653-008-01, 81653-008-02, 81653-008-03 - Packager: Guangzhou Jianyuan Biological Technology.Co.,Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 28, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Active IngredientMinoxidil 5% w/ v.
-
PurposeHair regrowth treatment for men and women
-
UseTo regrow hair
-
WarningsFor external use only. Flammable: Keep away from fire or flame
-
Do not useDo not use if : *You are a pregnant woman - *Your amount of hair loss is different than that shown on the ...
-
When Using ●Do not apply on other parts of the body - ●Avoid contact with the eyes. In case of accidental contact, rinse - eyes with large amounts of cool tap water. ●Some people have experienced ...
-
Stop Use.Chest pain, rapid heartbeat, faintness, or dizziness occurs. .Sudden, unexplained weight gain occurs. .Your hands or feet swell. . Scalp irritation or redness occurs. .Unwanted ...
-
Ask DoctorIf have Allergy, plz check with doctor
-
Keep Oot Of Reach Of ChildrenYes, Keep Oot Of Reach Of Children
-
Directions●Men: Apply half a capful 2 times a day to the hair loss area. ●Women: Apply half a capful 1 time a day to the hair loss area. ●Massage to area with fingers, then wash hands well ...
-
Other information.Hair growth has been shown in a clinical study of men (mostly - white) aged 18-49 years who used it for 4 months. ·Store at controlled room temperature 20°to 25°C (68° to 77°F) contents ...
-
Inactive ingredientsAqua,hyaluronic acid, Cocamidopropyl betaine,keratin amino acids,tocopherol (vitamin E), glycerol, xanthan gum, Glyoxyloyl , allantoin, aloe barbadensis leaf extract, propylene glycol, vitas ...
-
QuestionsVisit www.regenivade.com - (732) 893-0603
-
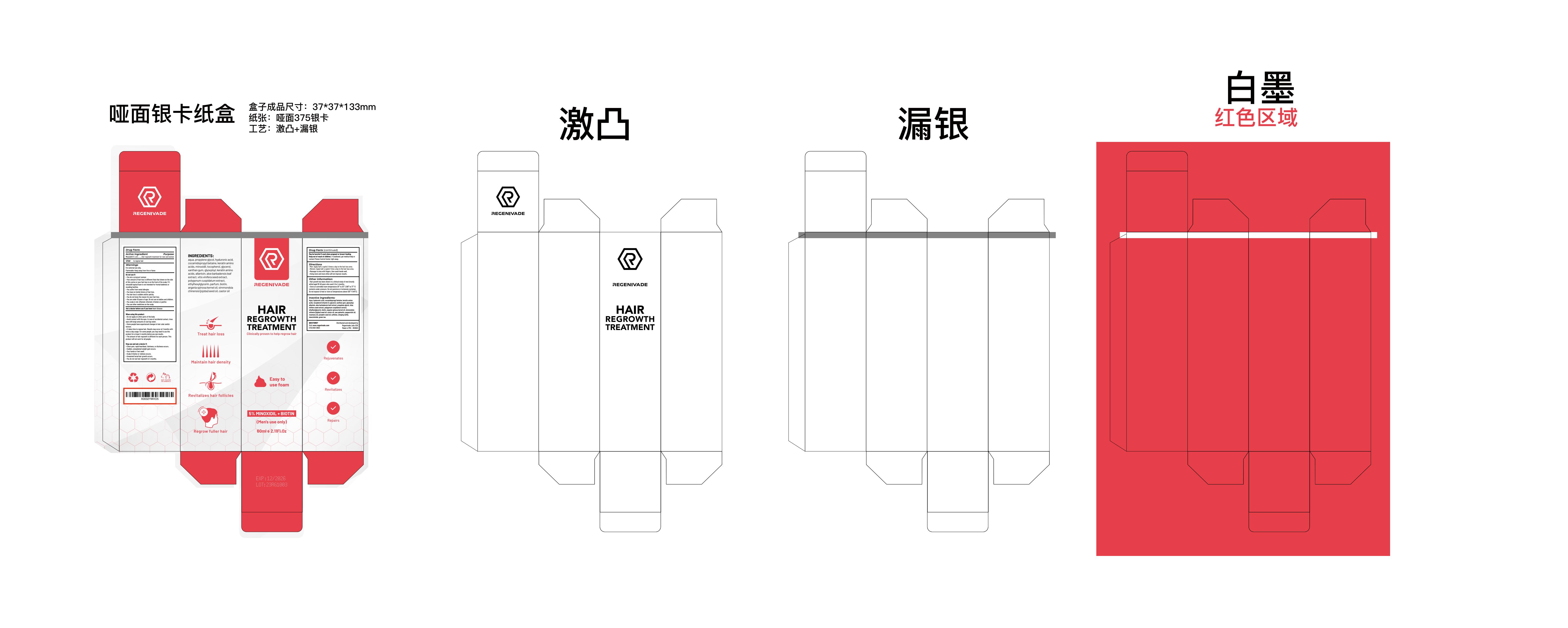
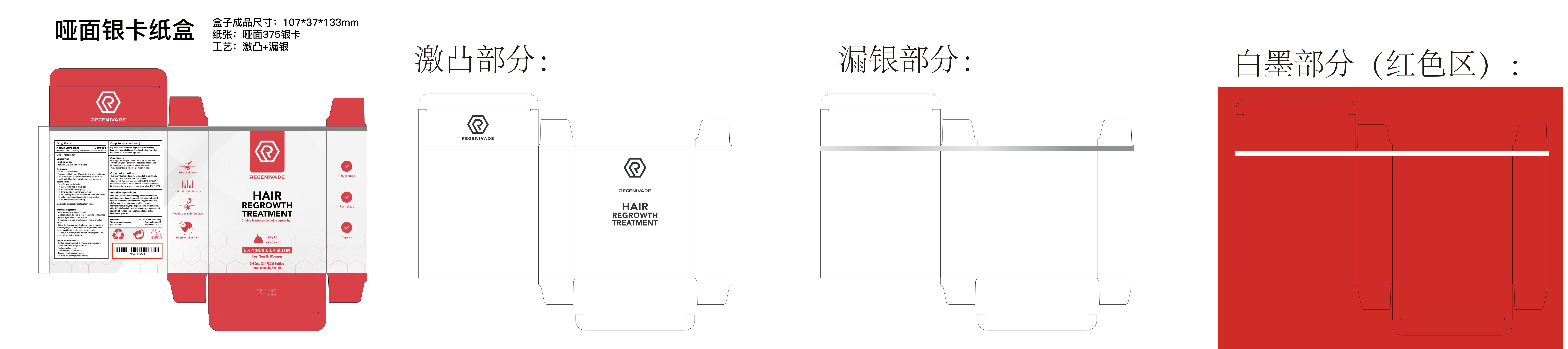
PRINCIPAL DISPLAY PANEL
 ...
... -
INGREDIENTS AND APPEARANCEProduct Information