Label: IQIRVO- elafibranor tablet, film coated
- NDC Code(s): 15054-0080-1
- Packager: Ipsen Biopharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated June 10, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use IQIRVO® safely and effectively. See full prescribing information for IQIRVO. IQIRVO (elafibranor) tablets, for oral use - Initial ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEIQIRVO is indicated for the treatment of primary biliary cholangitis (PBC) in combination with ursodeoxycholic acid (UDCA) in adults who have had an inadequate response to UDCA, or as monotherapy ...
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Evaluation Before Initiating IQIRVO - Before initiating IQIRVO: Evaluate for muscle pain or myopathy [see Warnings and Precautions (5.1)]. Verify that females of reproductive ...
-
3 DOSAGE FORMS AND STRENGTHSTablets: 80 mg, round, orange, film-coated tablets, debossed with "ELA 80" on one side and plain on the other side.
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Myalgia, Myopathy, and Rhabdomyolysis - Rhabdomyolysis resulting in acute kidney injury occurred in one IQIRVO-treated patient who had cirrhosis at baseline and was also taking a stable dose ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described elsewhere in the labeling: Myalgia, Myopathy, and Rhabdomyolysis [see Warnings and Precautions (5.1)] Fractures [see Warnings ...
-
7 DRUG INTERACTIONS7.1 Effects of IQIRVO on Other Drugs - Table 3 includes clinically significant drug interactions affecting other drugs. Table 3: Clinically Significant Interactions Affecting Other ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Based on data from animal reproduction studies, IQIRVO may cause fetal harm when administered during pregnancy. Treatment of pregnant rats with elafibranor ...
-
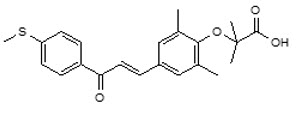
11 DESCRIPTIONElafibranor and its main active metabolite GFT1007 are peroxisome proliferator-activated receptor (PPAR) agonists. Elafibranor is practically insoluble in aqueous media at pH in the range 1.2 to ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Elafibranor and its main active metabolite GFT1007 are peroxisome proliferator-activated receptor (PPAR) agonists, both of which activate PPAR-alpha, PPAR-gamma, and ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - In a 2-year study in CD-1 mice, oral administration of elafibranor produced hepatocellular tumors (adenoma or ...
-
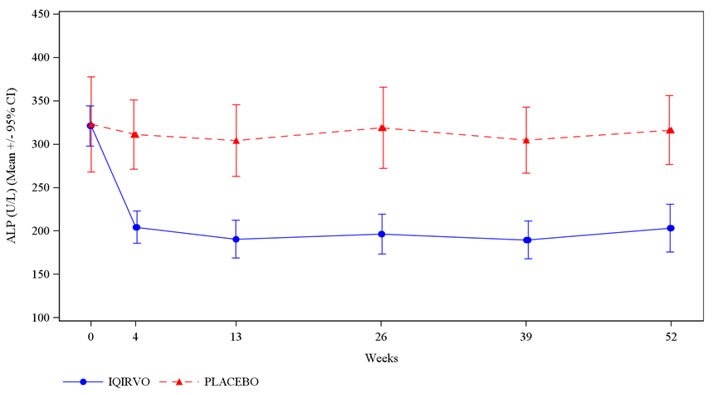
14 CLINICAL STUDIESThe efficacy of IQIRVO was evaluated in Study 1 (NCT04526665), a multi-center, randomized, double-blind, placebo-controlled study. The study included 161 adults with PBC with an inadequate ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGHow Supplied - IQIRVO (elafibranor) tablets are available as 80 mg, round, orange, film-coated tablets, debossed with 'ELA 80' on one side and plain on the other side. IQIRVO is supplied in a ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Medication Guide). Myalgia, Myopathy, and Rhabdomyolysis - Advise patients that IQIRVO may cause rhabdomyolysis. Inform patients to ...
-
SPL UNCLASSIFIED SECTIONManufactured for: Ipsen Biopharmaceuticals, Inc., One Main Street, 7th Floor, Cambridge, MA, 02142, USA
-
MEDICATION GUIDEThis Medication Guide has been approved by the U.S. Food and Drug AdministrationApproved: 06/2024 MEDICATION GUIDE - IQIRVO® (eye-ker-vo) (elafibranor) tablets - What is ...
-
PRINCIPAL DISPLAY PANEL - 80 mg Tablet Bottle CartonRx only - NDC 15054-0080-1 - IQIRVO® (elafibranor) tablets - 80 mg - For Oral Use - 30 - tablets
-
INGREDIENTS AND APPEARANCEProduct Information