Label: RIVFLOZA- nedosiran injection, solution
- NDC Code(s): 0169-5306-10, 0169-5307-08, 0169-5308-01
- Packager: Novo Nordisk
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated March 3, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use RIVFLOZA safely and effectively. See full prescribing information for RIVFLOZA. RIVFLOZA® (nedosiran) injection, for subcutaneous ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE RIVFLOZA is indicated to lower urinary oxalate levels in children 2 years of age and older and adults with primary hyperoxaluria type 1 (PH1) and relatively preserved kidney function, e.g., eGFR ...
-
2 DOSAGE AND ADMINISTRATION 2.1 Recommended Dosage - RIVFLOZA is administered subcutaneously once monthly at the recommended doses shown in Table 1. Dosing is based on actual body weight. Table 1: RIVFLOZA Dose Regimen in ...
-
3 DOSAGE FORMS AND STRENGTHS RIVFLOZA Injection 160 mg/mL (present as 170 mg nedosiran sodium) is a clear, colorless-to-yellow solution available as follows: • 80 mg/0.5 mL single-dose vial - • 128 mg/0.8 mL single-dose ...
-
4 CONTRAINDICATIONS None.
-
6 ADVERSE REACTIONS 6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
8 USE IN SPECIFIC POPULATIONS 8.1 Pregnancy - Risk Summary - Available data from reports of pregnancy in clinical trials with RIVFLOZA are insufficient to evaluate for a drug-associated risk of major birth defects ...
-
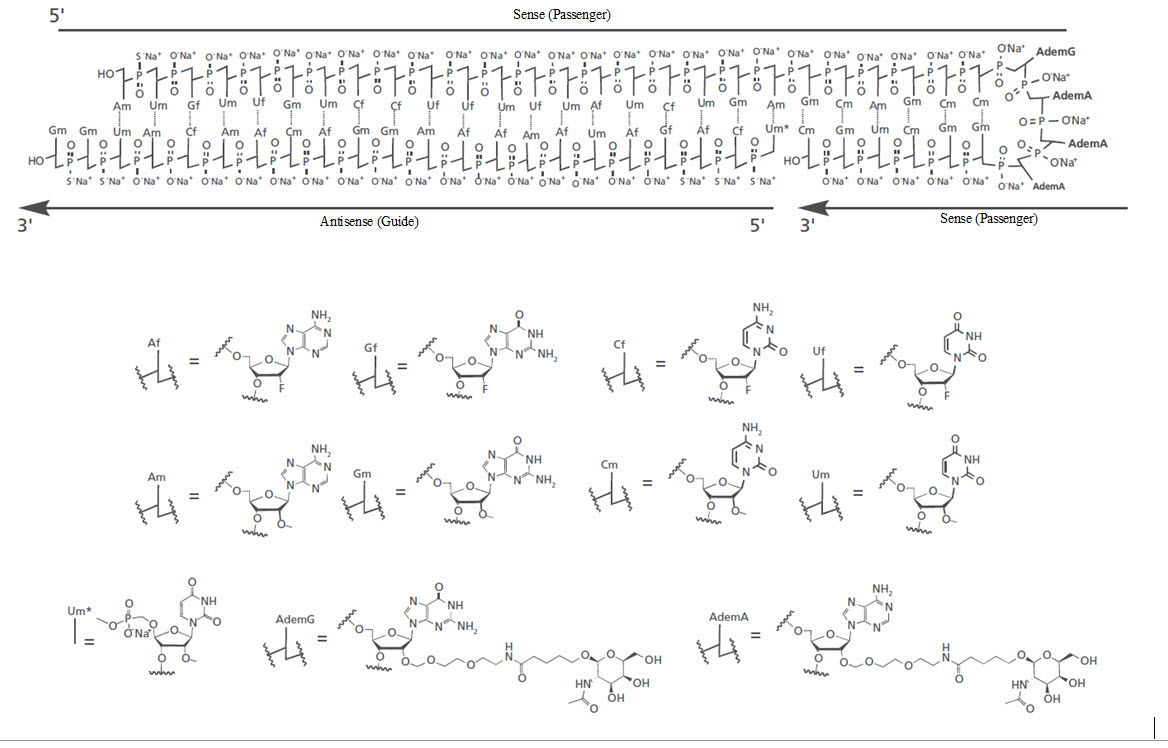
11 DESCRIPTION RIVFLOZA injection contains nedosiran, a double-stranded small interfering RNA (siRNA) with four covalently attached N-acetyl-D-galactosamine (GalNAc) residues. Nedosiran targets lactate ...
-
12 CLINICAL PHARMACOLOGY 12.1 Mechanism of Action - Nedosiran is a double-stranded siRNA, conjugated to GalNAc aminosugar residues. After subcutaneous administration, the GalNAc-conjugated sugars bind to ...
-
13 NONCLINICAL TOXICOLOGY 13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenicity - Long-term studies to assess carcinogenic risk of nedosiran have not been conducted. Genotoxicity - Nedosiran was not ...
-
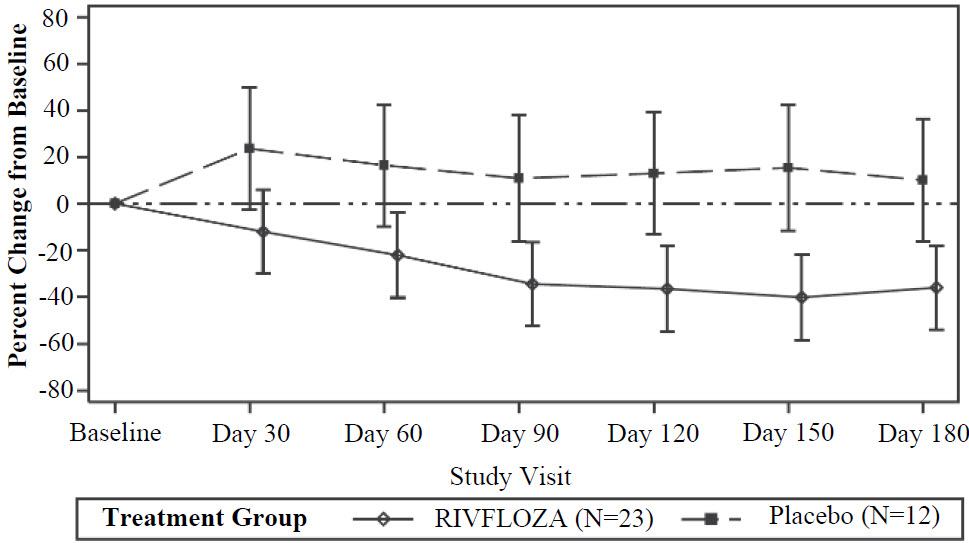
14 CLINICAL STUDIES 14.1 PHYOX2 - PHYOX2 was a randomized, double-blind trial comparing RIVFLOZA and placebo in patients aged 6 years or older with PH1 or PH2 and an eGFR ≥ 30 mL/min/1.73 m2 (NCT03847909). Too few ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING 16.1 How Supplied - RIVFLOZA is a clear, sterile, preservative-free, colorless-to-yellow solution available in single-dose pre-filled syringes and single-dose vials in cartons containing one ...
-
17 PATIENT COUNSELING INFORMATION Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use). • Instruct patients/caregivers on the appropriate dose of RIVFLOZA to use, the ...
-
Patient Package Insert PATIENT INFORMATION - RIVFLOZA® (Riv-flo-za) (nedosiran) injection, for subcutaneous use - What is RIVFLOZA? RIVFLOZA is a prescription medicine used to lower urinary oxalate levels ...
-
Instructions for Use – Pre-filled Syringe INSTRUCTIONS FOR USE - RIVFLOZA® (Riv-flo-za) (nedosiran) injection, for subcutaneous use - Single-dose Pre-filled Syringe - This Instructions for Use contains information on how ...
-
Instructions for Use - Vial INSTRUCTIONS FOR USE - RIVFLOZA® (Riv-flo-za) (nedosiran) injection, for subcutaneous use - Single-dose vial - This Instructions for Use contains information on how to inject RIVFLOZA ...
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – Pre-filled Syringe 128 mg/0.8 mL NDC: 0169-5307-08 - List 530708 - rivfloza™ (nedosiran) injection - 128 mg/0.8 mL - For subcutaneous injection only - 1 x 0.8 mL Sterile Single-dose Pre-filled Syringe - Do not use the Pre-filled Syringe ...
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – Pre-filled Syringe 160 mg/mL NDC: 0169-5306-10 - List 530610 - rivfloza™ (nedosiran) injection - 160 mg/mL - For subcutaneous injection only - 1 x 1 mL Sterile Single-dose Pre-filled Syringe - Do not use the Pre-filled Syringe if the ...
-
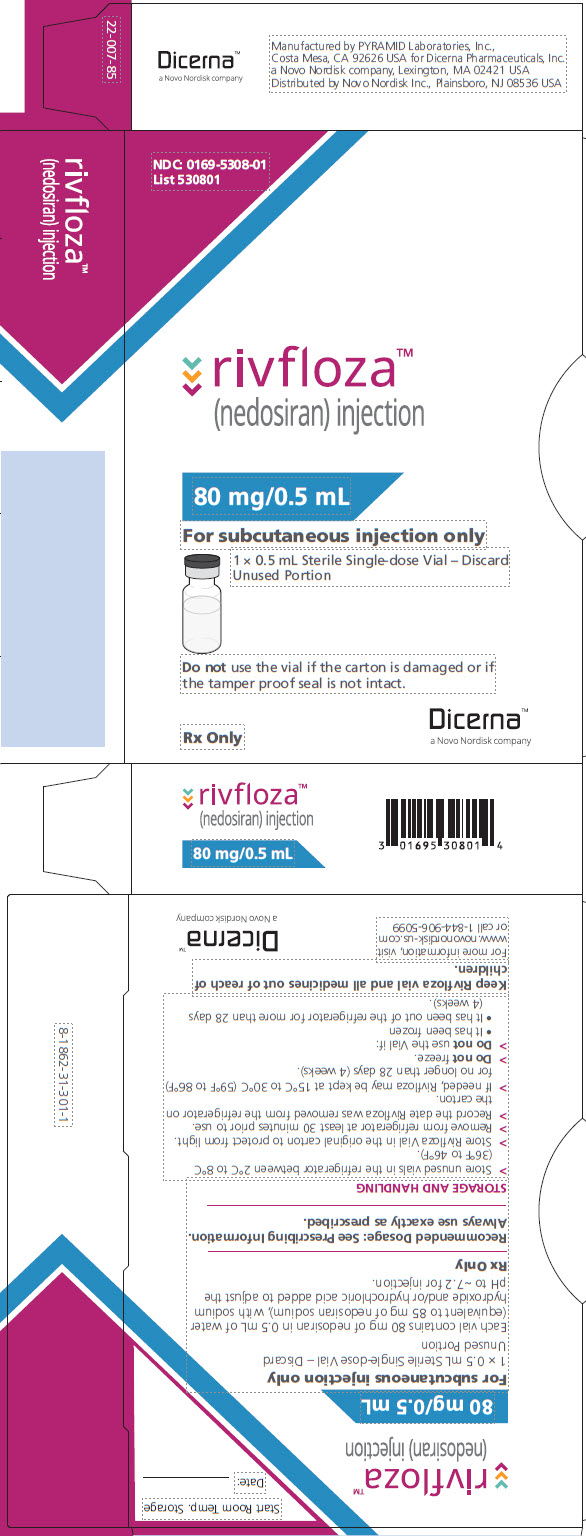
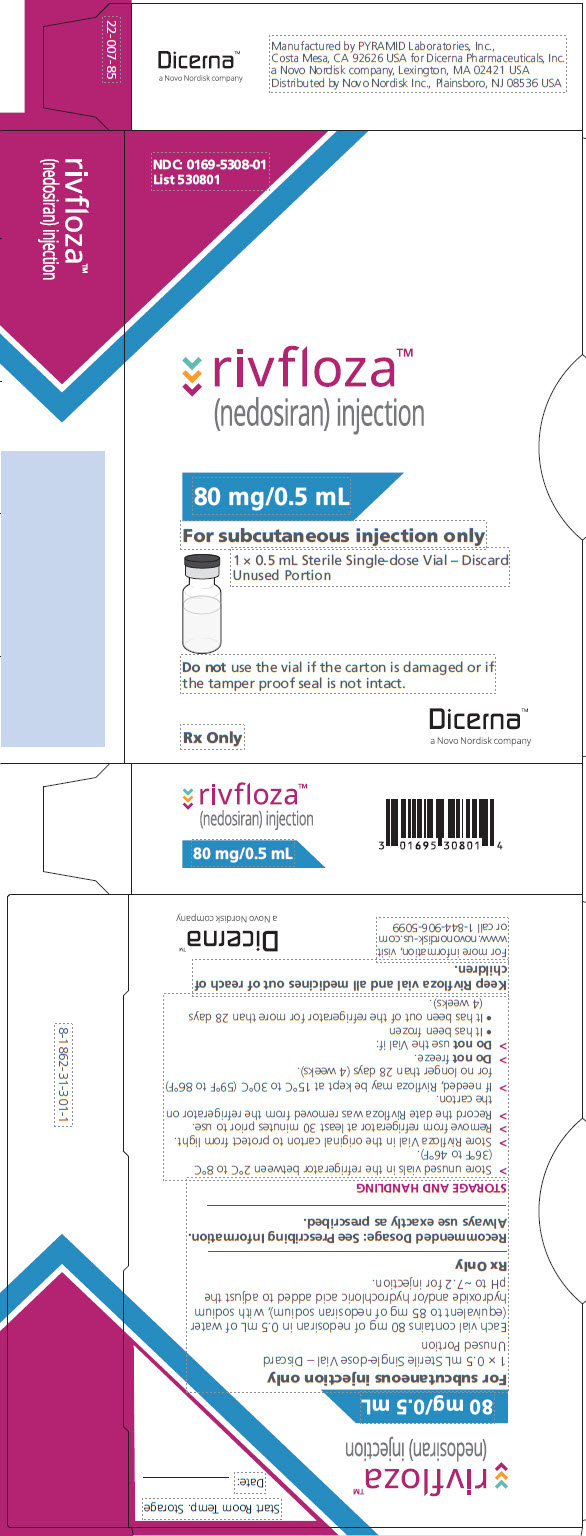
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – Vial 80 mg/0.5 mL NDC: 0169-5308-01 - List 530801 - rivfloza™ (nedosiran) injection - 80 mg/0.5 mL - For subcutaneous injection only - 1 x 0.5 mL Sterile Single-dose Vial – Discard Unused Portion - Do not use the vial if ...
-
INGREDIENTS AND APPEARANCEProduct Information




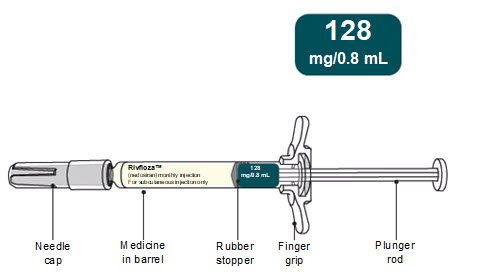
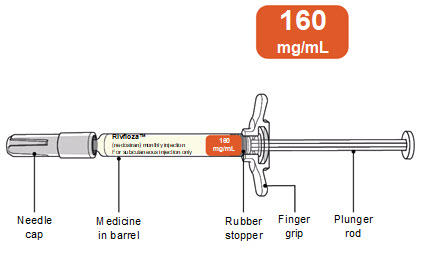
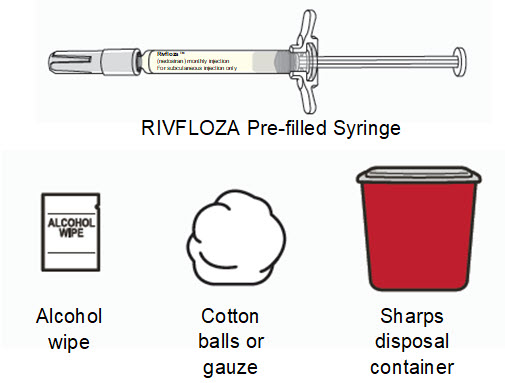
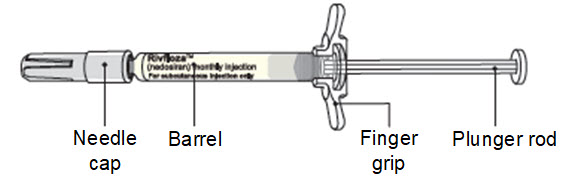
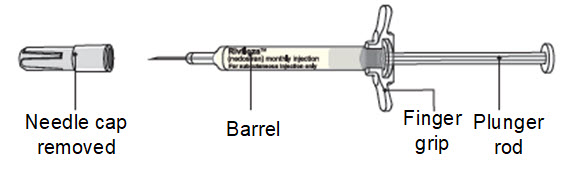
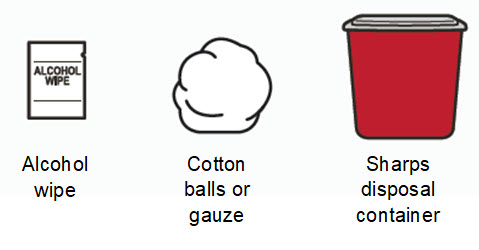
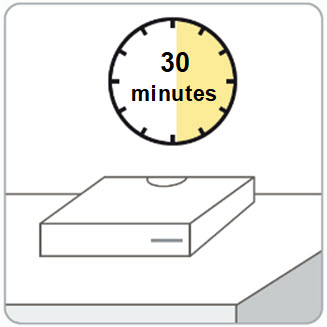
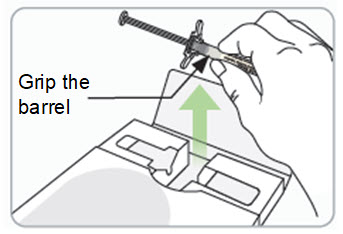
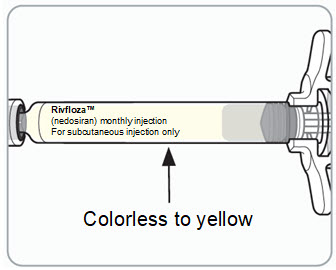
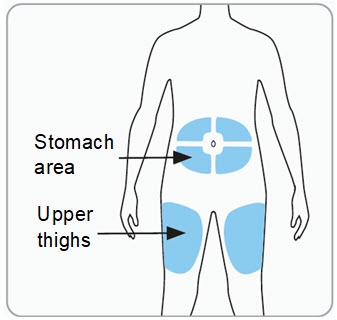
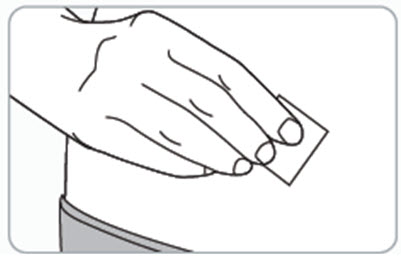
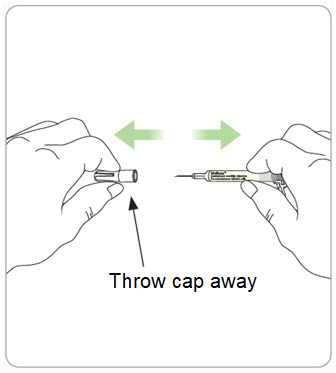
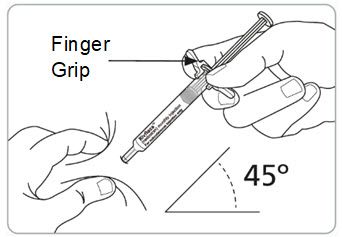
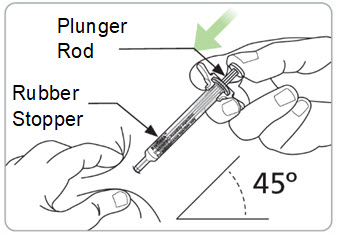
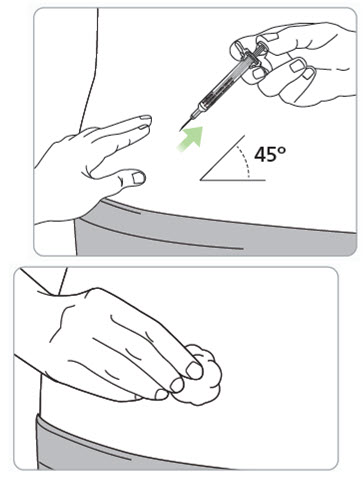
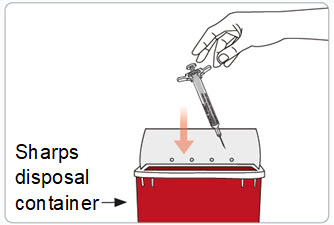


 Read Entire Instructions Before Use
Read Entire Instructions Before Use Follow Instructions Carefully
Follow Instructions Carefully Contact Novo Nordisk for any Questions
Contact Novo Nordisk for any Questions


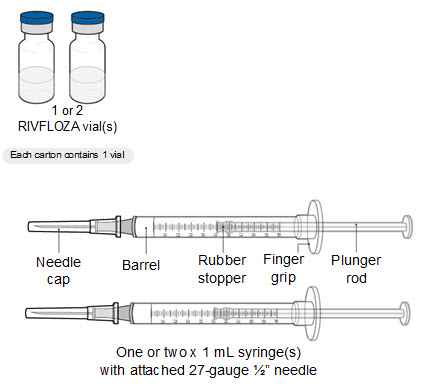
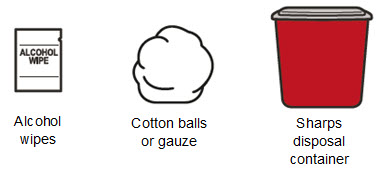
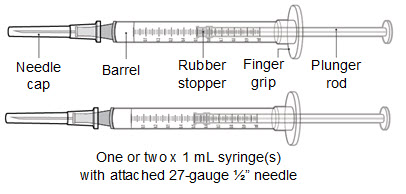
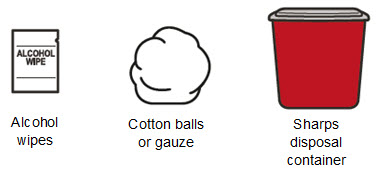
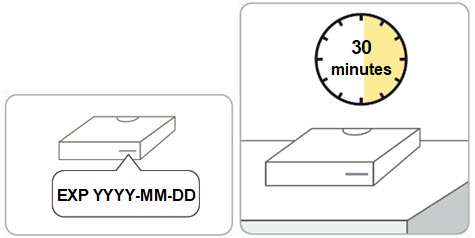
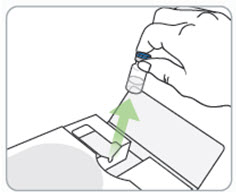
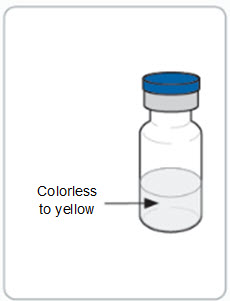
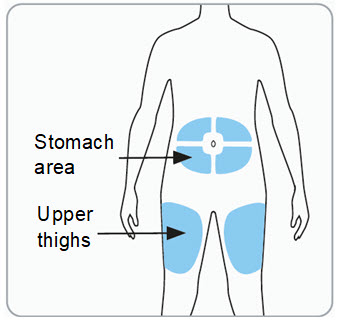
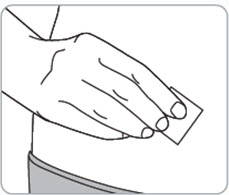
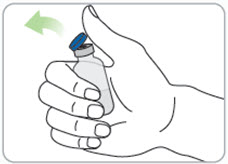
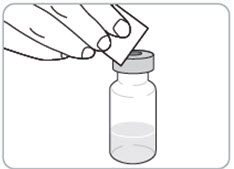
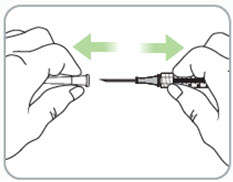
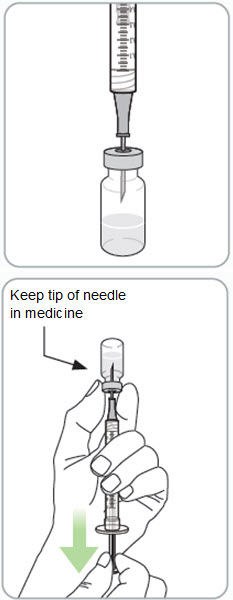
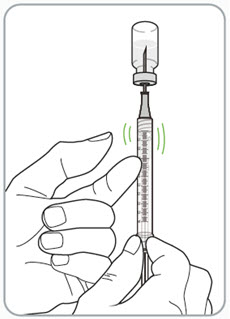
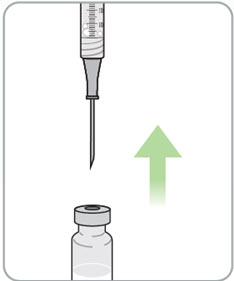
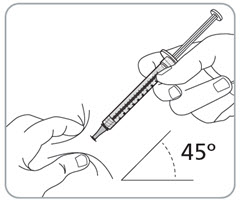
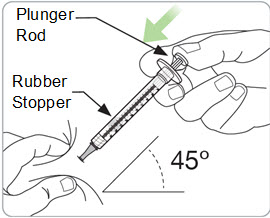
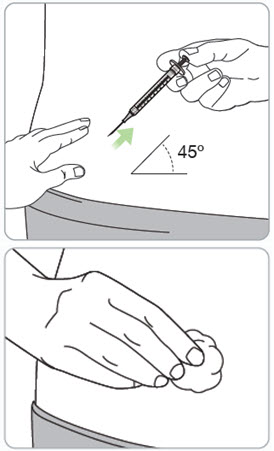
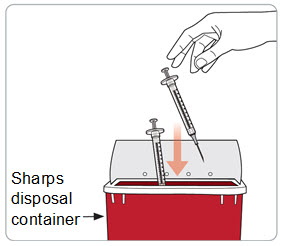


 Read Entire Instructions Before Use
Read Entire Instructions Before Use Follow Instructions Carefully
Follow Instructions Carefully Contact Novo Nordisk for any Questions
Contact Novo Nordisk for any Questions