Label: WINREVAIR- sotatercept-csrk kit
-
NDC Code(s):
0006-5068-99,
0006-5069-99,
0006-5079-89,
0006-5080-89, view more0006-5087-01, 0006-5088-01, 0006-5090-01, 0006-5091-01
- Packager: Merck Sharp & Dohme LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated May 1, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use WINREVAIR safely and effectively. See full prescribing information for WINREVAIR. WINREVAIR™ (sotatercept-csrk) for injection, for ...These highlights do not include all the information needed to use WINREVAIR safely and effectively. See full prescribing information for WINREVAIR.
WINREVAIR™ (sotatercept-csrk) for injection, for subcutaneous use
Initial U.S. Approval: 2024INDICATIONS AND USAGE
WINREVAIR is an activin signaling inhibitor indicated for the treatment of adults with pulmonary arterial hypertension (PAH, WHO Group 1) to increase exercise capacity, improve WHO functional class (FC) and reduce the risk of clinical worsening events. (1)
DOSAGE AND ADMINISTRATION
- The recommended starting dose is 0.3 mg/kg by subcutaneous injection. (2.1)
- The recommended target dose is 0.7 mg/kg every 3 weeks by subcutaneous injection. (2.2)
- Dosage modifications due to increased hemoglobin (Hgb) and decreased platelets may be necessary. Check Hgb and platelets before each dose for the first 5 doses, or longer if values are unstable, and monitor periodically thereafter. (2.3)
- See full prescribing information for preparation and administration instructions. (2.4)
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
None (4)
WARNINGS AND PRECAUTIONS
- Erythrocytosis: If severe, may increase the risk of thromboembolic events and hyperviscosity syndrome. Monitor Hgb before each dose for the first 5 doses, or longer if values are unstable, and periodically thereafter to determine if dose adjustments are required. (5.1)
- Severe Thrombocytopenia: May increase the risk of bleeding. Monitor platelets before each dose for the first 5 doses, or longer if values are unstable, and periodically thereafter to determine if dose adjustments are required. (5.2)
- Serious Bleeding: Serious bleeding events were reported and were more likely with concomitant prostacyclin and/or antithrombotic agents, or with low platelet counts. Do not administer WINREVAIR if the patient is experiencing serious bleeding. (5.3)
- Embryo-Fetal Toxicity: May cause fetal harm. Advise females of reproductive potential of the potential risk to a fetus and use of effective contraception. (5.4, 8.1, 8.3)
- Impaired Fertility: May impair female and male fertility. (5.5, 8.3, 13.1)
ADVERSE REACTIONS
The most common (≥10% in patients receiving WINREVAIR and 5% more than placebo) adverse reactions were headache, epistaxis, rash, telangiectasia, diarrhea, dizziness, and erythema. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Merck Sharp & Dohme LLC at 1-877-888-4231 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
USE IN SPECIFIC POPULATIONS
Lactation: Breastfeeding not recommended. (8.2)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 5/2025
Close -
Table of ContentsTable of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Starting Dosage
2.2 Recommended Target Dosage
2.3 Dosage Modifications Due to Hemoglobin Increase or Platelet Count Decrease
2.4 Preparation and Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Erythrocytosis
5.2 Severe Thrombocytopenia
5.3 Serious Bleeding
5.4 Embryo-Fetal Toxicity
5.5 Impaired Fertility
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.6 Immunogenicity
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Pulmonary Arterial Hypertension
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGEWINREVAIR™ is indicated for the treatment of adults with pulmonary arterial hypertension (PAH, World Health Organization [WHO] Group 1) to increase exercise capacity, improve WHO functional class ...
WINREVAIR™ is indicated for the treatment of adults with pulmonary arterial hypertension (PAH, World Health Organization [WHO] Group 1) to increase exercise capacity, improve WHO functional class (FC), and reduce the risk of clinical worsening events.
Close -
2 DOSAGE AND ADMINISTRATION2.1 Recommended Starting Dosage - WINREVAIR is administered once every 3 weeks by subcutaneous injection according to patient body weight. The starting dose of WINREVAIR is 0.3 mg/kg. Obtain ...
2.1 Recommended Starting Dosage
WINREVAIR is administered once every 3 weeks by subcutaneous injection according to patient body weight. The starting dose of WINREVAIR is 0.3 mg/kg.
Obtain hemoglobin (Hgb) and platelet count prior to the first dose of WINREVAIR. Do not initiate treatment if platelet count is <50,000/mm3 (<50 x 109/L) [see Dosage and Administration (2.3)].
Injection volume for starting dose is calculated based on patient weight as follows:
Injection Volume (mL) = Weight (kg) x 0.3 mg/kg 50 mg/mL Injection volume should be rounded to the nearest 0.1 mL.
For example: (70 kg x 0.3 mg/kg) ÷ 50 mg/mL = 0.42 mL, rounds to 0.4 mL
See Table 1 for selecting the appropriate kit based on calculated injection volume for starting dose.
Table 1: Kit Type Based on Injection Volume for Dose of 0.3 mg/kg Injection Volume (mL) Kit Type 0.2 to 0.9 45 mg kit (containing 1 x 45 mg vial) 1 to 1.1 60 mg kit (containing 1 x 60 mg vial) 2.2 Recommended Target Dosage
After verifying acceptable Hgb and platelet count, increase to the target dose of 0.7 mg/kg. Continue treatment at 0.7 mg/kg every 3 weeks unless dosage adjustments are required [see Dosage and Administration (2.3)].
Injection volume for target dose is calculated based on patient weight as follows:
Injection Volume (mL) = Weight (kg) x 0.7 mg/kg 50 mg/mL Injection volume should be rounded to the nearest 0.1 mL.
For example: (70 kg x 0.7 mg/kg) ÷ 50 mg/mL = 0.98 mL, rounds to 1 mL
See Table 2 for selecting the appropriate kit based on calculated injection volume for target dose.
Table 2: Kit Type Based on Injection Volume for Dose of 0.7 mg/kg Injection Volume (mL) Kit Type 0.4 to 0.9 45 mg kit (containing 1 x 45 mg vial) 1 to 1.2 60 mg kit (containing 1 x 60 mg vial) 1.3 to 1.8 90 mg kit (containing 2 x 45 mg vials) 1.9 to 2.4 120 mg kit (containing 2 x 60 mg vials) Missed Dose, Overdose, and Underdose
If a dose of WINREVAIR is missed, administer as soon as possible. If the missed dose of WINREVAIR is not administered within 3 days of the scheduled date, adjust the schedule to maintain 3-week dosing intervals. In case of an overdose, monitor for erythrocytosis [see Overdosage (10)].
2.3 Dosage Modifications Due to Hemoglobin Increase or Platelet Count Decrease
Check Hgb and platelet count before each dose for the first 5 doses, or longer if values are unstable. Thereafter, monitor Hgb and platelet count periodically [see Warnings and Precautions (5.1, 5.2)].
Delay treatment for at least 3 weeks if any of the following occur:
- Hgb increases >2.0 g/dL from the previous dose and is above ULN.
- Hgb increases >4.0 g/dL from baseline.
- Hgb increases >2.0 g/dL above ULN.
- Platelet count decreases to <50,000/mm3 (<50 x 109/L).
Recheck Hgb and platelet count before reinitiating treatment. For treatment delays lasting >9 weeks, restart treatment at 0.3 mg/kg, and escalate to 0.7 mg/kg after verifying acceptable Hgb and platelet count.
Close2.4 Preparation and Administration
Administration is subject to monitoring of hemoglobin and platelet count [see Dosage and Administration (2.3), Warnings and Precautions (5.1, 5.2)].
WINREVAIR is intended for use under the guidance of a healthcare professional. Patients and caregivers may administer WINREVAIR when considered appropriate and when they receive training and follow-up from the healthcare provider (HCP) on how to reconstitute, prepare, measure, and inject WINREVAIR [see Patient Counseling Information (17)].
Confirm at subsequent visits that the patient and/or caregiver can correctly prepare and administer WINREVAIR, particularly if the dose changes or the patient requires a different kit [see Warnings and Precautions (5.1)].
Refer to the Instructions for Use (IFU) for detailed instructions on the proper preparation and administration of WINREVAIR.
Selecting the Appropriate Product Kit
If a patient’s body weight requires the use of two 45 mg vials or two 60 mg vials of lyophilized product, use a 2-vial kit instead of two individual 1-vial kits. A 2-vial kit includes instructions to combine the contents of two vials, which aids in measuring the proper dosage and eliminates the need for multiple injections [see How Supplied/Storage and Handling (16.1)].
Reconstitution Instructions
- Remove the injection kit from the refrigerator and wait 15 minutes to allow the prefilled syringe(s) and drug product to come to room temperature prior to preparation.
- Attach the vial adapter to the vial.
- Visually inspect the pre-filled syringe for any damage or leaks and the Sterile Water for Injection inside to ensure there are no visible particles.
- Snap off the cap of the pre-filled syringe and attach the syringe to the vial adapter.
- Inject all of the Sterile Water for Injection from the attached syringe into the vial containing the lyophilized powder. This will provide a final concentration of 50 mg/mL.
- Gently swirl the vial to reconstitute the drug product. DO NOT shake or vigorously agitate.
- Allow the vial to stand for up to 3 minutes to allow bubbles to disappear.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
- When properly mixed, WINREVAIR should be clear to opalescent and colorless to slightly brownish-yellow and does not have clumps or powder.
- If prescribed a 2-vial presentation, repeat the steps within this section to prepare the second vial.
- Use the reconstituted solution as soon as possible, but no later than 4 hours after reconstitution. Discard unused reconstituted solution.
Syringe Preparation
- Turn the syringe and vial upside-down and withdraw the appropriate volume for injection, based on the patient’s weight.
- If the dose amount requires the use of two vials, withdraw the entire contents of the first vial and slowly transfer full contents into the second vial.
- Turn the syringe and vial upside-down and withdraw the required amount of drug product.
- If necessary, remove excess drug product.
- If necessary, remove excess air from the syringe.
Administration Instructions
WINREVAIR is for subcutaneous injection.
- Select the injection site on the abdomen (at least 2 inches away from navel), upper thigh, or upper arm, and swab with an alcohol wipe. Select a new site for each injection that is not scarred, tender, or bruised.
- For administration by the patient or caregiver, use only the abdomen and upper thigh (see IFU).
- Perform subcutaneous injection.
-
3 DOSAGE FORMS AND STRENGTHSFor injection: 45 mg white to off-white lyophilized cake or powder appearance in a single-dose vial. For injection: 60 mg white to off-white lyophilized cake or powder appearance in a single-dose ...
- For injection: 45 mg white to off-white lyophilized cake or powder appearance in a single-dose vial.
- For injection: 60 mg white to off-white lyophilized cake or powder appearance in a single-dose vial.
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Erythrocytosis - WINREVAIR may increase hemoglobin. Severe erythrocytosis may increase the risk of thromboembolic events or hyperviscosity syndrome. In clinical studies, moderate elevations ...
5.1 Erythrocytosis
WINREVAIR may increase hemoglobin. Severe erythrocytosis may increase the risk of thromboembolic events or hyperviscosity syndrome. In clinical studies, moderate elevations in Hgb (>2 g/dL above ULN) occurred in 15% of patients taking WINREVAIR while no elevations ≥4 g/dL above ULN were observed. Monitor Hgb before each dose for the first 5 doses, or longer if values are unstable, and periodically thereafter, to determine if dose adjustments are required [see Dosage and Administration (2.3), Adverse Reactions (6.1)].
5.2 Severe Thrombocytopenia
WINREVAIR may decrease platelet count. Severe thrombocytopenia may increase the risk of bleeding. In clinical studies, severe thrombocytopenia (platelet count <50,000/mm3 [<50 x 109/L]) occurred in 3% of patients taking WINREVAIR. Thrombocytopenia occurred more frequently in patients also receiving prostacyclin infusion.
Do not initiate treatment if platelet count is <50,000/mm3 [see Dosage and Administration (2.3)].
Monitor platelets before each dose for the first 5 doses, or longer if values are unstable, and periodically thereafter to determine whether dose adjustments are required. [see Dosage and Administration (2.3), Adverse Reactions (6.1)].
5.3 Serious Bleeding
In clinical studies, serious bleeding (e.g., gastrointestinal, intracranial hemorrhage) was reported in 4% of patients taking WINREVAIR and 1% of patients taking placebo. Patients with serious bleeding were more likely to be on prostacyclin background therapy and/or antithrombotic agents, or have low platelet counts. Advise patients about signs and symptoms of blood loss. Evaluate and treat bleeding accordingly. Do not administer WINREVAIR if the patient is experiencing serious bleeding [see Warnings and Precautions (5.2), Adverse Reactions (6.1)].
5.4 Embryo-Fetal Toxicity
Based on findings in animal reproduction studies, WINREVAIR may cause fetal harm when administered to a pregnant woman. In animal reproduction studies, administration of WINREVAIR to pregnant rats and rabbits during organogenesis resulted in adverse developmental outcomes, including increased embryo-fetal mortality, alterations to growth, and structural variations at exposures 4-fold and 0.6-fold (based on area under the curve [AUC]) those occurring at the maximum recommended human dose (MRHD), respectively. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use an effective method of contraception during treatment with WINREVAIR and for at least 4 months after the final dose [see Use in Specific Populations (8.1, 8.3)].
Close5.5 Impaired Fertility
Based on findings in animals, WINREVAIR may impair female and male fertility. Advise patients on the potential effects on fertility [see Use in Specific Populations (8.3), Nonclinical Toxicology (13.1)].
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described elsewhere in the labeling: Erythrocytosis [see Warnings and Precautions (5.1)] Severe Thrombocytopenia [see Warnings and ...
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Erythrocytosis [see Warnings and Precautions (5.1)]
- Severe Thrombocytopenia [see Warnings and Precautions (5.2)]
- Serious Bleeding [see Warnings and Precautions (5.3)]
- Embryo-Fetal Toxicity [see Warnings and Precautions (5.4)]
- Impaired Fertility [see Warnings and Precautions (5.5)]
Close6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The following data reflect exposure to WINREVAIR in the STELLAR trial. Patients (n=323) were randomized in a 1:1 ratio to receive WINREVAIR or placebo in combination with background standard of care therapies. Patients received a starting dose of 0.3 mg/kg via SC injection and the dose was increased to the target dose of 0.7 mg/kg administered once every 3 weeks for 24 weeks. After completing the primary 24-week treatment phase, patients continued into a long-term double-blind (LTDB) treatment period, maintaining their randomized treatment assignment, until all patients completed the primary treatment period. The median duration of treatment was 273 days in the placebo group and 313 days in the WINREVAIR group [see Clinical Studies (14.1)].
The most common adverse reactions occurring in STELLAR (≥10% for WINREVAIR and at least 5% more than placebo) are shown in Table 3.
Table 3: Adverse Reactions ≥10% in Patients Receiving WINREVAIR and at least 5% More Than Placebo in STELLAR* Adverse reaction Placebo
N=160WINREVAIR
N=163- *
- Double-blind placebo-controlled period + Long-term double-blind period of STELLAR
Headache 28 (17.5) 40 (24.5) Epistaxis 3 (1.9) 36 (22.1) Rash 13 (8.1) 33 (20.2) Telangiectasia 7 (4.4) 27 (16.6) Diarrhea 16 (10.0) 25 (15.3) Dizziness 10 (6.2) 24 (14.7) Erythema 5 (3.1) 22 (13.5) Increased Hemoglobin
Increases in Hgb were managed by dose delays (10%), dose reductions (6%), or both (5%). Shifts in Hgb from normal to above normal levels occurred in 87 (53%) patients receiving WINREVAIR and in 23 (14%) patients receiving placebo.
Thrombocytopenia
Decreases in platelets were managed by dose delays (2%), dose reductions (2%), or both (2%). Shifts in platelet count from normal to below normal occurred in 40 (25%) patients receiving WINREVAIR and in 26 (16%) patients receiving placebo.
Telangiectasia
In patients exposed to WINREVAIR who experienced telangiectasia, the median time to onset was 47.1 weeks.
Increased Blood Pressure
In patients taking WINREVAIR, mean systolic/diastolic blood pressure increased from baseline by 2.2/4.9 mmHg at 24 weeks. In patients taking placebo, the change from baseline in mean blood pressure was -1.6/-0.6 mmHg.
Treatment Discontinuation
The incidences of treatment discontinuations due to an adverse reaction were 4% in the WINREVAIR group and 7% in the placebo group. No specific adverse reactions causing treatment discontinuations occurred with a frequency greater than 1% and more often in the WINREVAIR group.
Uncontrolled Long-term Safety Data
The safety profile in the long-term uncontrolled extension period of the PULSAR study was generally similar to that observed in the STELLAR study. Patients were treated with WINREVAIR 0.3 mg/kg or 0.7 mg/kg (n=104) and had a mean duration of exposure of 151 weeks (maximum 218 weeks).
In SOTERIA, an ongoing open-label study of the long-term safety and efficacy of WINREVAIR, right-to-left intrapulmonary shunting has been reported in 2 participants (<0.5%) who developed worsening hypoxemia despite improved PAH hemodynamics.
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Based on findings in animal reproduction studies, WINREVAIR may cause fetal harm when administered to a pregnant woman. There are risks to the mother and the fetus ...
8.1 Pregnancy
Risk Summary
Based on findings in animal reproduction studies, WINREVAIR may cause fetal harm when administered to a pregnant woman. There are risks to the mother and the fetus associated with pulmonary arterial hypertension in pregnancy (see Clinical Considerations). There are no available data on WINREVAIR use in pregnant women to inform a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes.
In animal reproduction studies, administration of WINREVAIR to pregnant rats and rabbits during the period of organogenesis resulted in adverse developmental outcomes, including embryo-fetal mortality, alterations to growth, and structural variations at exposures 4-fold and 0.6-fold (based on area under the curve [AUC]) above those occurring at the maximum recommended human dose (MRHD), respectively (see Data). Advise pregnant women of the potential risk to a fetus [see Use in Specific Populations (8.3)].
The background risk of major birth defects and miscarriage for the indicated population is not known. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Report exposure during pregnancy or lactation to the Merck Sharp & Dohme, LLC Adverse Event reporting line at 1-877-888-4231.
Disease-Associated Maternal and/or Embryo/Fetal Risk
In patients with pulmonary arterial hypertension, pregnancy is associated with an increased rate of maternal and fetal morbidity and mortality, including spontaneous abortion, intrauterine growth restriction, and premature labor.
Animal Data
In embryo-fetal developmental toxicity studies, pregnant animals were dosed subcutaneously with sotatercept-csrk during the period of organogenesis. Sotatercept-csrk was administered to rats on gestation days 6 and 13 at doses of 5, 15, or 50 mg/kg and to rabbits on gestation days 7 and 14 at doses of 0.5, 1.5, or 5 mg/kg. Effects in both species included reductions in numbers of live fetuses and fetal body weights, delays in ossification, and increases in resorptions and post-implantation losses. In rats and rabbits, these effects were observed at exposures (based on area under the curve [AUC]) approximately 4-fold and 0.6-fold the maximum recommended human dose (MRHD), respectively. In rats only, skeletal variations (increased number of supernumerary ribs and changes in the number of thoracic or lumbar vertebrae) occurred at an exposure 15-fold the human exposure at the MRHD.
In a prenatal and postnatal development study in rats, sotatercept-csrk was administered subcutaneously at doses of 1.5 and 5 mg/kg on gestation days 6 and 13, or at dosages of 1.5, 5, or 10 mg/kg during lactation on days 1, 8, and 15. There were no adverse effects in first filial generation (F1) pups from dams dosed during gestation at estimated exposures up to 2-fold the MRHD. In F1 pups from dams dosed during lactation, decreases in pup weight correlated with delays in sexual maturation at estimated exposures (based on AUC) ≥2-fold the MRHD.
8.2 Lactation
Risk Summary
There are no data on the presence of sotatercept-csrk in human milk, the effects on the breastfed infant, or the effects on milk production. Because of the potential for serious adverse reactions in the breastfed child, advise patients that breastfeeding is not recommended during treatment with WINREVAIR, and for 4 months after the final dose.
8.3 Females and Males of Reproductive Potential
WINREVAIR may cause fetal harm when administered to pregnant women [see Use in Specific Populations (8.1)].
Pregnancy Testing
Pregnancy testing is recommended for females of reproductive potential before starting WINREVAIR treatment.
Contraception
Females
Advise female patients of reproductive potential to use effective contraception during treatment with WINREVAIR and for at least 4 months after the final dose if treatment is discontinued [see Use in Specific Populations (8.1)].
Infertility
Based on findings in animals, sotatercept-csrk may impair female and male fertility [see Nonclinical Toxicology (13.1)]. In male rats, although adverse histologic changes in reproductive organs were not reversible after a 13-week period, functional fertility demonstrated reversibility.
8.4 Pediatric Use
The safety and effectiveness of WINREVAIR have not been established in patients less than 18 years of age.
Close8.5 Geriatric Use
A total of 81 patients ≥65 years of age participated in clinical studies for PAH, of which 52 (16%) were treated with WINREVAIR. No differences in efficacy of WINREVAIR were observed between the <65-year-old and ≥65-year-old subgroups.
With the exception of bleeding events (a collective group of adverse events of clinical interest), there were no differences in safety between the <65-year-old and ≥65-year-old subgroups. Bleeding events occurred more commonly in the older WINREVAIR subgroup, but with no imbalance between age subgroups for any specific bleeding event.
Clinical studies of WINREVAIR did not include sufficient numbers of patients aged 75 and older to determine whether they respond differently from younger patients.
-
10 OVERDOSAGEIn healthy volunteers, WINREVAIR dosed at 1 mg/kg resulted in increases in Hgb associated with hypertension; both improved with phlebotomy. In the event of overdose, monitor closely for increases ...
In healthy volunteers, WINREVAIR dosed at 1 mg/kg resulted in increases in Hgb associated with hypertension; both improved with phlebotomy. In the event of overdose, monitor closely for increases in Hgb and blood pressure, and provide supportive care as appropriate. WINREVAIR is not dialyzable.
Close -
11 DESCRIPTIONSotatercept-csrk is a homodimeric recombinant fusion protein consisting of the extracellular domain of the human activin receptor type IIA (ActRIIA) linked to the human IgG1 Fc domain. The ...
Sotatercept-csrk is a homodimeric recombinant fusion protein consisting of the extracellular domain of the human activin receptor type IIA (ActRIIA) linked to the human IgG1 Fc domain. The molecular weight based on the amino acid sequence of sotatercept-csrk is approximately 78 kDa as a homodimer.
Sotatercept-csrk for injection is a sterile, preservative-free, white to off-white lyophilized cake or powder appearance in single-dose vials for subcutaneous administration after reconstitution.
Each 45 mg single-dose vial provides 45 mg of sotatercept-csrk and citric acid monohydrate (0.40 mg), polysorbate 80 (0.18 mg), sodium citrate (1.84 mg), and sucrose (72 mg) at pH 5.8. After reconstitution with 1 mL Sterile Water for Injection, the resulting concentration is 50 mg/mL of sotatercept-csrk and the nominal deliverable volume is 0.9 mL.
Each 60 mg single-dose vial provides 60 mg of sotatercept-csrk and citric acid monohydrate (0.53 mg), polysorbate 80 (0.24 mg), sodium citrate (2.45 mg), and sucrose (96 mg) at pH 5.8. After reconstitution with 1.3 mL Sterile Water for Injection, the resulting concentration is 50 mg/mL of sotatercept-csrk and the nominal deliverable volume is 1.2 mL.
Close -
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Sotatercept-csrk, a recombinant activin receptor type IIA-Fc (ActRIIA-Fc) fusion protein, is an activin signaling inhibitor that binds to activin A and other TGF- ...
12.1 Mechanism of Action
Sotatercept-csrk, a recombinant activin receptor type IIA-Fc (ActRIIA-Fc) fusion protein, is an activin signaling inhibitor that binds to activin A and other TGF- β superfamily ligands. As a result, sotatercept-csrk improves the balance between the pro-proliferative (ActRIIA/Smad2/3-mediated) and anti-proliferative (BMPRII/Smad1/5/8-mediated) signaling to modulate vascular proliferation. In rat models of PAH, a sotatercept-csrk analog reduced inflammation and inhibited proliferation of endothelial and smooth muscle cells in diseased vasculature. These cellular changes were associated with thinner vessel walls, partial reversal of right ventricular remodeling, and improved hemodynamics.
12.2 Pharmacodynamics
PVR:
A statistically significantly greater decrease from baseline in PVR was observed in the WINREVAIR group compared to the placebo group in the Phase 3 STELLAR study. The median treatment difference in PVR between sotatercept-csrk and placebo was -235 dynes*sec/cm5 (95% CI: -288, -181; p<0.001). Sotatercept-csrk steady state exposure at 0.7 mg/kg dose was associated with near maximal reduction in PVR based on exposure-response analysis.
NT-proBNP:
A statistically significantly greater decrease from baseline in NT-proBNP was observed in the WINREVAIR group compared to the placebo group in the Phase 3 STELLAR study. The median treatment difference in NT-proBNP between the sotatercept-csrk and placebo was -442 pg/mL (95% CI: -574, -310; p<0.001).
12.3 Pharmacokinetics
Following subcutaneous administration of 0.7 mg/kg WINREVAIR every three weeks to PAH patients, the steady state geometric mean (%CV) area under the time concentration curve (AUC) is 172 mcg×d/mL (34.2%), and peak concentration (Cmax) is 9.7 mcg/mL (30%). Sotatercept-csrk AUC and Cmax increased proportionally with dose. Steady state is achieved after approximately 15 weeks following initiation of multiple dosing. The accumulation ratio of sotatercept-csrk AUC is approximately 2.2.
Absorption
Following subcutaneous administration, the absolute bioavailability of sotatercept-csrk is approximately 66%. The sotatercept-csrk median time to peak drug concentration (Tmax) is approximately 7 days (range from 2 to 8 days) following multiple SC administration every 4 weeks.
Distribution
The population PK model estimated volume of distribution (%CV) of sotatercept-csrk at steady state is approximately 5.3 L (27.3%) in patients with PAH.
Elimination
The sotatercept-csrk effective half-life is approximately 24 days and its clearance is approximately 0.18 L/day.
Metabolism
Sotatercept-csrk is expected to be metabolized into small peptides by catabolic pathways.
Specific Populations
No clinically significant differences in sotatercept-csrk pharmacokinetics (PK) were observed based on age (18 to 81 years of age), sex, race, mild to moderate (eGFR ranging from 30 to 89 mL/min) renal impairment (PAH patients), or end-stage kidney disease (eGFR <15 mL/min) with dialysis. Severe renal impairment (eGFR ranging from 15 to 30 mL/min) is not expected to impact the PK of sotatercept-csrk. Sotatercept-csrk is not dialyzable. The effect of hepatic impairment on the PK of sotatercept-csrk has not been studied.
Body Weight
The clearance (CL) and central volume of distribution (Vc) increase with increase in body weight. This effect is not clinically significant when sotatercept-csrk is administered using weight-based dosing as recommended.
Close12.6 Immunogenicity
The observed incidence of anti-drug antibodies is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of anti-drug antibodies in the study described below with the incidence of anti-drug antibodies in other studies, including those of WINREVAIR or of other sotatercept-csrk products.
During the 24-week treatment period in STELLAR, 27% (44/163) of sotatercept-csrk-treated patients developed anti-sotatercept-csrk antibodies (ADA). Among the 44 ADA-positive patients, 12 (27%) tested positive for neutralizing antibodies against sotatercept-csrk.
There were no identified clinical effects of anti-sotatercept-csrk antibodies on pharmacokinetics, pharmacodynamics, safety, or effectiveness of sotatercept-csrk over the treatment duration of 24 weeks at the recommended dosage.
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - No carcinogenicity or mutagenicity studies have been conducted with sotatercept-csrk. In a fertility and early embryonic development ...Close
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No carcinogenicity or mutagenicity studies have been conducted with sotatercept-csrk.
In a fertility and early embryonic development study in female rats, sotatercept-csrk was administered SC once weekly at doses of 5, 15, and 50 mg/kg beginning 2 weeks prior to mating and through gestation day 7. At doses ≥15 mg/kg (≥9 fold the MRHD, based on estimated AUC), pregnancy rates were decreased and there were increases in preimplantation and postimplantation loss and reductions in live litter size. Increased estrous cycle duration occurred at 50 mg/kg only (21-fold the MRHD, based on estimated AUC).
In a fertility study in male rats, sotatercept-csrk was administered SC once weekly at doses of 0.3, 3, and 30 mg/kg for 13 weeks (beginning 10 weeks prior to mating). A subset of animals was examined after a 13-week recovery period. At ≥0.3 mg/kg (0.5-fold the MRHD, based on estimated AUC) there were non-reversible histologic changes in the efferent ducts, testes, and epididymides. Reversible decreases in functional fertility endpoints occurred at 30 mg/kg (20-fold the MRHD, based on estimated AUC).
-
14 CLINICAL STUDIES14.1 Pulmonary Arterial Hypertension - The efficacy of WINREVAIR was evaluated in adult patients with PAH in the STELLAR trial (NCT04576988). STELLAR was a global, double-blind ...Close
14.1 Pulmonary Arterial Hypertension
The efficacy of WINREVAIR was evaluated in adult patients with PAH in the STELLAR trial (NCT04576988). STELLAR was a global, double-blind, placebo-controlled, multicenter, parallel-group clinical trial in which 323 patients with PAH (WHO Group 1 FC II or III) were randomized 1:1 to WINREVAIR (target dose 0.7 mg/kg) (n=163) or placebo (n=160) administered subcutaneously once every 3 weeks.
Participants were: 79% female; had a median age of 48 years (range: 18 to 82 years), and median body weight of 68 kg (range 38 to 141 kg); and 89% White/Caucasian, 2% Black/African American, 2% Asian, 0.3% American Indian or Alaska Native, 0.3% Native Hawaiian or Other Pacific Islander, 6% Missing/Other races. The most common PAH etiologies were idiopathic PAH (59%), heritable PAH (18%), and PAH associated with connective tissue diseases (CTD) (15%). STELLAR excluded patients with human immunodeficiency virus (HIV)-associated PAH, PAH associated with portal hypertension, schistosomiasis-associated PAH, and pulmonary veno occlusive disease. The mean time from PAH diagnosis to screening was 8.8 years. Most participants were receiving either three (61%) or two (35%) background drugs for PAH, and 40% were receiving prostacyclin infusions. Patients had a WHO FC II (49%) or III (51%) at baseline.
The primary efficacy endpoint was the change from baseline at Week 24 in 6-Minute Walk Distance (6 MWD). In the WINREVAIR group, the placebo-adjusted median increase in 6 MWD was 41 meters (95% CI: 28, 54; p<0.001). Figure 1 displays placebo-adjusted changes in 6 MWD at Week 24 in relevant subgroups.
Figure 1: Change from Baseline in 6-Minute Walk Distance (meters) at Week 24 in Subgroups *Hodges-Lehmann location shift from placebo estimate (median of all paired differences). ASE = asymptotic standard error. 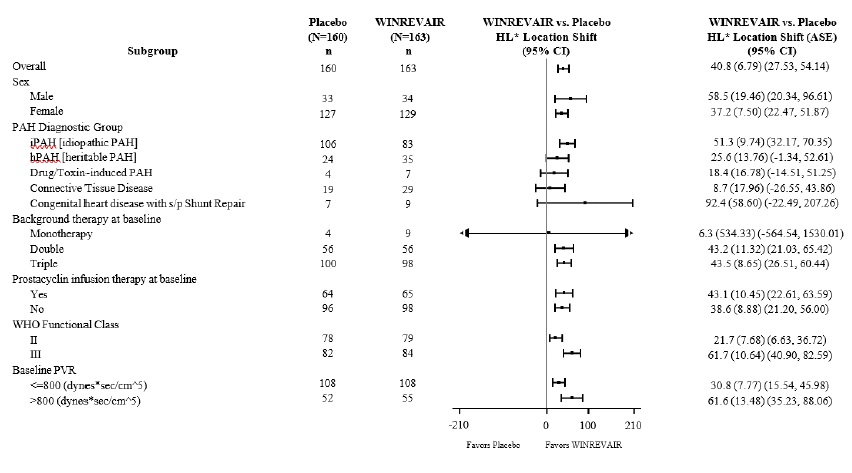
Change from baseline in 6 MWD at Week 24 for subjects who died was imputed to -2000 meters to receive the worst rank. Change from baseline in 6 MWD at Week 24 for subjects who had missing data due to a non-fatal clinical worsening event was imputed to -1000 meters to receive the next-worst rank.
Treatment with WINREVAIR led to an improvement from baseline by at least 1 WHO FC at Week 24 in 29% of patients compared to 14% of patients treated with placebo (p<0.001).
Treatment with WINREVAIR resulted in an 84% reduction in the occurrence of death from any cause or PAH clinical worsening events compared to placebo (see Table 4 and Figure 2). These outcomes were captured until the last patient completed the Week 24 visit (data up to the data cutoff; median duration of exposure 33.6 weeks).
Table 4: Death from Any Cause or PAH Clinical Worsening Events Placebo
(N=160)
n (%)WINREVAIR
(N=163)
n (%)Hazard Ratio
(95% CI)N = number of subjects in the category.
6 MWT = 6-Minute Walking Test- *
- A subject can have more than one assessment recorded for their clinical worsening.
- †
- There were no events of atrial septostomy.
- ‡
- Deterioration of PAH is defined by both of the following events occurring at any time, even if they began at different times, as compared to their baseline values: (a) Worsened WHO functional class (II to III, III to IV, II to IV, etc.); and (b) Decrease in 6 MWD by ≥15% (confirmed by two 6 MWTs at least 4 hours apart but no more than one week).
Number of subjects who experienced death or at least one clinical worsening event 42 (26.3) 9 (5.5) 0.16 (0.08, 0.35)
p<0.001Assessment of clinical worsening events* Death 7 (4.4) 2 (1.2) Worsening-related listing for lung and/or heart transplant 2 (1.3) 1 (0.6) Need to initiate rescue therapy with an approved PAH therapy or
the need to increase the dose of infusion prostacyclin by 10% or more17 (10.6) 2 (1.2) Need for atrial septostomy† 0 (0.0) 0 (0.0) PAH-specific hospitalization (≥24 hours) 8 (5.0) 0 (0.0) Deterioration of PAH‡ 15 (9.4) 4 (2.5) Figure 2: Time to Death from Any Cause or First Occurrence of PAH Clinical Worsening Event Kaplan-Meier Plot 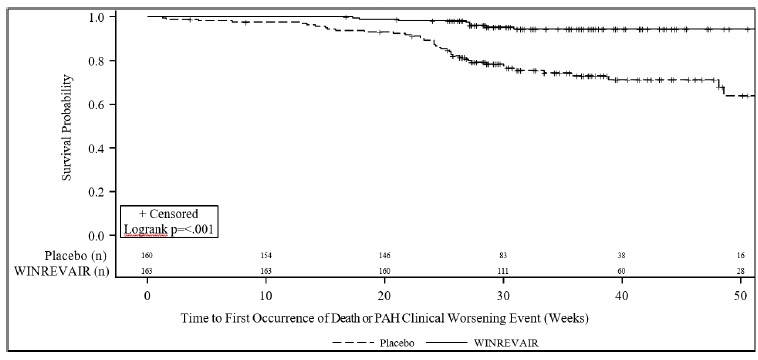
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - WINREVAIR (sotatercept-csrk) for injection is a white to off-white lyophilized cake or powder appearance supplied in single-dose vials (45 mg or 60 mg) packaged in kits that ...
16.1 How Supplied
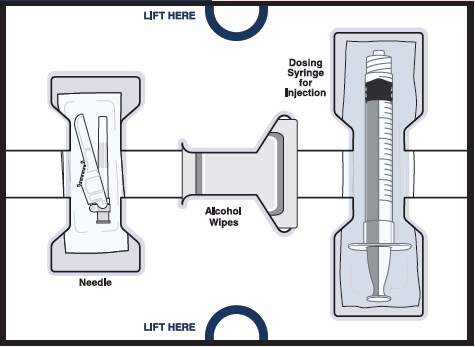
WINREVAIR (sotatercept-csrk) for injection is a white to off-white lyophilized cake or powder appearance supplied in single-dose vials (45 mg or 60 mg) packaged in kits that contain one measuring syringe and one safety needle. Each kit also contains Sterile Water for Injection in prefilled syringes necessary to reconstitute the product, vial adapter(s), and alcohol pads, as shown in Table 5.
Table 5: Kit Contents Kit Vial
AdaptersAlcohol
PadsSterile Water for Injection
in prefilled syringesNDC (1) 45 mg vial 1 4 (1) 1 mL syringe NDC # 0006-5090-01 (1) 60 mg vial 1 4 (1) 1.3 mL syringe NDC # 0006-5091-01 (2) 45 mg vials 2 8 (2) 1 mL syringes NDC # 0006-5087-01 (2) 60 mg vials 2 8 (2) 1.3 mL syringes NDC # 0006-5088-01 Close16.2 Storage and Handling
Store vials refrigerated at 2°C to 8°C (36°F to 46°F) in original carton to protect from light. Do not freeze.
The kit should remain in the refrigerator until ready for use. The unused kit can be out of the refrigerator for (up to 25°C/77°F) up to 24 hours. For additional information on temperature excursions, call Merck Sharp & Dohme LLC at 1-800-672-6372.
-
17 PATIENT COUNSELING INFORMATIONAdvise patients to read the FDA-approved patient labeling (Patient Information and IFU). Discuss the following with patients prior to and during treatment with WINREVAIR. Erythrocytosis - Caution ...
Advise patients to read the FDA-approved patient labeling (Patient Information and IFU).
Discuss the following with patients prior to and during treatment with WINREVAIR.
Erythrocytosis
Caution patients that WINREVAIR may raise Hgb to levels that increase their risk of thrombotic events. Inform patients that Hgb levels will be assessed before at least the first 5 doses and then periodically, as dosage may need to be adjusted [see Warnings and Precautions (5.1)].
Severe Thrombocytopenia
Caution patients that WINREVAIR may cause platelet count to decrease, which if severe could cause bleeding. Inform patients that platelet count will be assessed before at least the first 5 doses and then periodically, as dosage may need to be adjusted [see Warnings and Precautions (5.2)].
Serious Bleeding
Inform patients of the possibility of serious bleeding, which is more likely to occur if they have low platelet counts or while on prostacyclin background therapy and/or antithrombotic agents. Advise patients to notify their healthcare provider about signs and symptoms of bleeding [see Warnings and Precautions (5.3)].
Embryo-Fetal Toxicity
Advise females of reproductive potential of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception while receiving WINREVAIR and for at least 4 months after the final dose. Advise females to contact their healthcare provider if they become pregnant, or if pregnancy is suspected during treatment with WINREVAIR [see Warnings and Precautions (5.4), Use in Specific Populations (8.1)]. Report exposure during pregnancy or lactation to the Merck Sharp & Dohme, LLC Adverse Event reporting line at 1-877-888-4231.
Lactation
Advise females not to breastfeed during treatment with WINREVAIR and for 4 months after the final dose [see Use in Specific Populations (8.2)].
Females and Males of Reproductive Potential
Advise females and males of reproductive potential that WINREVAIR may impair fertility [see Use in Specific Populations (8.3), Nonclinical Toxicology (13.1)].
Administration by Patient or Caregiver
Review the IFU with the patient or caregiver step-by-step. Provide training to the patient or caregiver regarding proper preparation and administration of WINREVAIR and decide whether a patient or caregiver is capable of preparing and administering WINREVAIR independently [see Dosage and Administration (2.4)].
Make sure the patient or caregiver can do the following correctly:
- reconstitute the medicine,
- measure the correct amount of medicine according to the patient’s prescription,
- select and prepare a proper injection site, and
- inject the medicine subcutaneously.
Incorrect Dose or Missed Dose
Inform patients to call their healthcare provider for further instruction if they take more than or less than the correct dose. Advise them about signs/symptoms to monitor for and what to do if any of these signs/symptoms should occur. Advise them that additional laboratory tests may be required prior to the next scheduled dose to ensure that the next dose can be safely administered.
Instruct the patient that if they miss the prescribed dose of WINREVAIR, they should take it within 3 days and maintain the original schedule for the next dose. If not taken within 3 days, instruct them to call their healthcare provider for guidance [see Dosage and Administration (2.2)].
Manufactured by:
Merck Sharp & Dohme LLC, Rahway, NJ 07065, USAU.S. license number 0002
For patent information: www.msd.com/research/patent
Copyright © 2024-2025 Merck & Co., Inc., Rahway, NJ, USA, and its affiliates.
All rights reserved.uspi-mk7962-i-2505r001
Close -
PATIENT PACKAGE INSERTThis Patient Information has been approved by the U.S. Food and Drug AdministrationIssued: 3/2024 - PATIENT INFORMATION - WINREVAIR ...Close
This Patient Information has been approved by the U.S. Food and Drug Administration Issued: 3/2024 PATIENT INFORMATION
WINREVAIR™ (WIN-reh-vair)
(sotatercept-csrk)
for injection, for subcutaneous useWhat is WINREVAIR?
WINREVAIR™ is a prescription medicine used to treat adults with pulmonary arterial hypertension (PAH). PAH is a type of high blood pressure in the arteries of your lungs.
WINREVAIR can improve your ability to exercise, improve your ability to perform normal activities with fewer symptoms, and reduce the risk of your physical condition and symptoms worsening.
It is not known if WINREVAIR is safe and effective in children under 18 years of age.
What should I tell my healthcare provider before taking WINREVAIR?
- Tell your healthcare provider about:
- All of your medical conditions
-
All of the medicines you take, including:
- Prescriptions
- Over-the-counter medicines
- Vitamins
- Herbal supplements
If you are able to get pregnant:
- WINREVAIR may harm your unborn baby. Tell your healthcare provider if you are pregnant or planning to get pregnant.
- Tell your healthcare provider right away if you become pregnant or think you may be pregnant during treatment with WINREVAIR.
- Your healthcare provider should do a pregnancy test before you start taking WINREVAIR.
- You should use effective birth control during treatment with WINREVAIR and for at least 4 months after your final dose. Ask your healthcare provider about birth control methods that may be right for you.
- You or your healthcare provider should report any exposure to WINREVAIR during pregnancy by calling 1-877-888-4231.
If you are breastfeeding or planning to breastfeed:
- Tell your healthcare provider if you are breastfeeding or plan to breastfeed. It is not known if WINREVAIR passes into breast milk.
- Do not breastfeed during treatment with WINREVAIR and for 4 months after your final dose. Talk to your healthcare provider about the best way to feed your baby.
- You or your healthcare provider should report any exposure to WINREVAIR during breastfeeding by calling 1-877-888-4231.
Before you take WINREVAIR: - If your healthcare provider decides that you or a caregiver can give your injections of WINREVAIR at home, you or your caregiver should receive training from your healthcare provider on the right way to prepare and inject WINREVAIR. Do not try to inject WINREVAIR until you have been shown how to inject WINREVAIR by your healthcare provider.
- Your healthcare provider will do a blood test before your first 5 doses of WINREVAIR, longer if needed, and then from time to time to check your levels of hemoglobin (a protein in red blood cells that carries oxygen) and platelets (blood cells that help blood clot). After each of these blood tests, your healthcare provider may delay treatment or change your dose if needed.
- See the detailed “Instructions for Use” booklet that comes with WINREVAIR for information on how to prepare and inject a dose of WINREVAIR.
How should I take WINREVAIR?
- Use WINREVAIR exactly as your healthcare provider tells you to.
- You will give WINREVAIR every 3 weeks as an injection just under your skin (subcutaneous) in your:
- stomach (abdomen) at least 2 inches away from the belly button, or
- upper thigh
- You should inject WINREVAIR right away after mixing the medicine powder with the sterile water for injection, but no later than 4 hours after mixing.
Your prescribed dose:
- Your healthcare provider will tell you how much WINREVAIR to inject and when to inject it. This is because your prescribed dose depends on your body weight and blood tests.
- Do not change your dose or stop taking WINREVAIR without talking to your healthcare provider.
- Do not take WINREVAIR more often than your healthcare provider tells you to. If you are not sure when to take WINREVAIR, call your healthcare provider.
- Your healthcare provider may delay treatment, change your dose, or stop treatment depending on how you respond to WINREVAIR.
What if I take the wrong dose or miss my dose?
- If you give more than or less than your prescribed dose, call your healthcare provider right away.
- If you miss your dose of WINREVAIR:
- If it has been 3 days or fewer, give it as soon as possible. You can then follow your usual schedule for your next dose.
- If it has been more than 3 days, call your healthcare provider for what to do.
What are the possible side effects of WINREVAIR?
WINREVAIR may cause serious side effects including:
- High level of hemoglobin in your blood. High levels of hemoglobin can increase your risk for blood clots. Your healthcare provider will do blood tests to check your hemoglobin levels before starting and regularly during treatment with WINREVAIR.
- Severely low number of platelets in your blood. Your healthcare provider will do blood tests to check your platelet levels before starting and regularly during treatment with WINREVAIR. Tell your healthcare provider if you develop easy bruising or bleeding, bleeding that does not stop, or nosebleeds.
- Serious bleeding. Tell your healthcare provider if you develop any signs or symptoms of bleeding, including:
- vomiting blood or your vomit looks like coffee-grounds
- pink or brown urine
- red or black stools that look like tar
- coughing up blood or blood clots
- persistent headaches
- nausea
- dizziness or feeling weak
- persistent abdominal cramps
- severe back pain
- abnormally heavy menstrual bleeding
- Decreased fertility: WINREVAIR may affect female and male fertility, which may affect your ability to have children. Talk to your healthcare provider if this is a concern for you.
The most common side effects of WINREVAIR include:
- headache
- nose bleeds
- rash
- tiny blood vessels that look like pink or red lines on the skin (spider veins)
- diarrhea
- dizziness
- redness
These are not all the possible side effects of WINREVAIR.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store WINREVAIR?
- Store WINREVAIR in the refrigerator at 36°F to 46°F (2°C to 8°C). Do not freeze.
- Store in the original carton to protect from light.
Keep WINREVAIR and all medicines out of the reach of children and pets.
General information about the safe and effective use of WINREVAIR
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use WINREVAIR for a condition for which it was not prescribed. Do not give WINREVAIR to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about WINREVAIR that is written for healthcare professionals.
What are the ingredients in WINREVAIR?
Active ingredient: sotatercept-csrk
Inactive ingredient(s): citric acid monohydrate, polysorbate 80, sodium citrate, and sucrose.Manufactured by: Merck Sharp & Dohme LLC, Rahway, NJ 07065, USA.
US License Number 0002
For patent information: www.msd.com/research/patent
Copyright © 2024 Merck & Co., Inc., Rahway, NJ, USA, and its affiliates.
All rights reserved.
usppi-mk7962-i-2403r000
For more information, call 1-877-888-4231, or go to www.WINREVAIR.com. - Tell your healthcare provider about:
-
INSTRUCTIONS FOR USEImportant: Read this booklet first - Important - INSTRUCTIONS FOR USE - Mix - WINREVAIR™ (WIN-reh-vair) (sotatercept-csrk) for injection, for subcutaneous use ...
Important: Read this booklet first
Important
INSTRUCTIONS FOR USE
Mix
WINREVAIR™ (WIN-reh-vair)
(sotatercept-csrk)
for injection, for subcutaneous use
How to use your WINREVAIR injection kit
Withdraw
 For package containing 1 vial
For package containing 1 vial
For subcutaneous injection only (inject directly under the skin)
InjectIn this booklet:
Important information for patients or caregivers
Get to know the parts of your kit
Important information you need to know before injecting WINREVAIR
 Mix powdered medicine into liquid form
Mix powdered medicine into liquid form

Read this booklet
Read this Instructions for Use from start to finish before using WINREVAIR and each time you get a refill. There may be new information.
Start with your healthcare provider
Do not use WINREVAIR until your healthcare provider has shown you or your caregiver the right way to prepare and inject it. Your healthcare provider should show you how to inject WINREVAIR before you use it for the first time.
Questions?
If you have questions about how to give WINREVAIR the right way or need more information, call your pharmacy or healthcare provider.
For general product information visit www.WINREVAIR.com or call Merck Sharp & Dohme LLC at 1-800-672-6372.Before you prepare and inject WINREVAIR, you must be:
- trained by a healthcare provider following this Instructions for Use booklet step-by-step, and
- capable of independent administration of WINREVAIR as determined by a healthcare provider
Before using WINREVAIR, make sure you are trained to do the following correctly:
- Reconstitute (mix) the medicine
- Measure the correct amount of medicine according to your prescription
- Select and prepare a proper injection site
- Inject the medicine subcutaneously
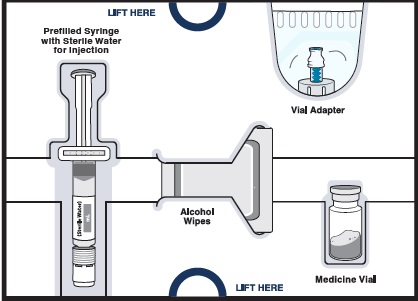
Get to know the parts of your kit
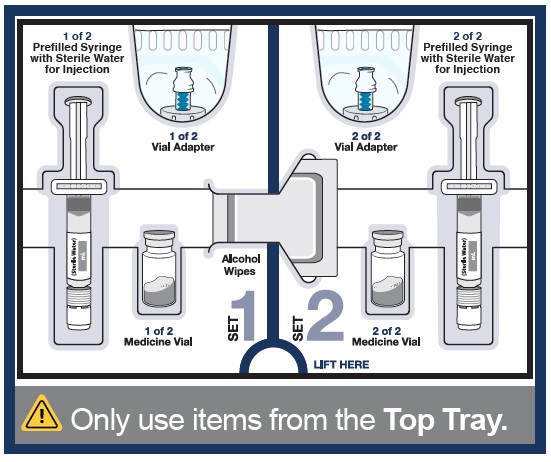
Top Tray
Use to mix the medicine
 1 Vial of WINREVAIR medicine powder
1 Vial of WINREVAIR medicine powder
 1 Prefilled syringe with sterile water for injection to mix medicine powder into liquid form
1 Prefilled syringe with sterile water for injection to mix medicine powder into liquid form 1 Vial adapter to connect the vial and syringe
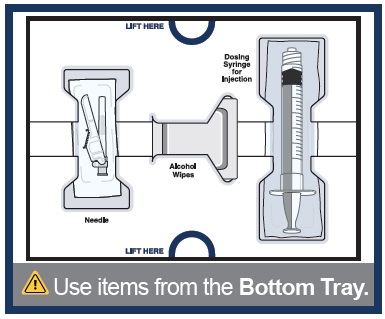
1 Vial adapter to connect the vial and syringeBottom Tray
Use to inject the medicine
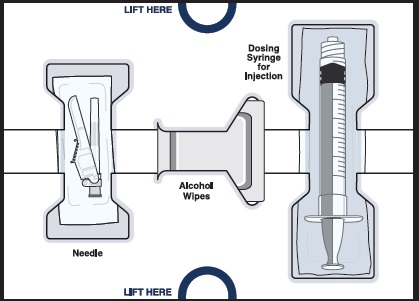
Needle for injection
Empty dosing syringe to measure, withdraw, and inject your medicineImportant information you need to know before injecting WINREVAIR
- You must mix WINREVAIR before using it. Make sure the medicine powder in the vial is completely dissolved before you inject.
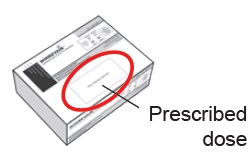
- Check your prescribed dose (amount in ‘mL’) each time you use WINREVAIR. Your prescribed dose may change. Your prescribed dose is on the prescription label on your kit.
- Use only the supplies that are in the kit to prepare your prescribed dose.
- Do not open the package or mix the medicine until you are ready to use it.
- Do not reuse any of the supplies. After your injection, throw away the used vials with any remaining WINREVAIR medicine, needle, caps, and syringes in a sharps container. See pages 36-37 for more information.
Store WINREVAIR injection kit in the refrigerator at 36°F to 46°F (2°C to 8°C). Do not freeze.
Store in the original carton to protect from light.

Keep injection kit out of the reach of children and pets.
Before you can prepare and inject WINREVAIR, you must first be trained and determined to be capable of independent administration of WINREVAIR by a healthcare provider.
1 Check WINREVAIR injection kit and expiration date
Remove the WINREVAIR injection kit from the refrigerator. 
 Check the expiration date and look for any signs of damage on the kit or on the supplies.
Check the expiration date and look for any signs of damage on the kit or on the supplies.
 If expired or damaged, do not use.
If expired or damaged, do not use.
Call your pharmacy to get a new kit. Check that you have the medicine that your healthcare provider prescribed.
Check that you have the medicine that your healthcare provider prescribed.2 Let your kit come to room temperature, gather supplies, and wash your hands
Wait 15 minutes to allow your kit to warm to room temperature.
Cold medicine is more painful to inject.

Along with your kit, gather these items and find a clean, flat surface where you will prepare and inject your dose.
 Sharps disposal container
Sharps disposal container Gauze, cotton ball, or bandage
Gauze, cotton ball, or bandageWash your hands with soap and water.
Mix powdered medicine into liquid form
Start with the Top Tray
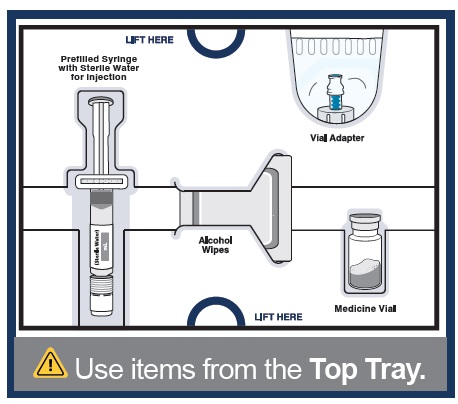
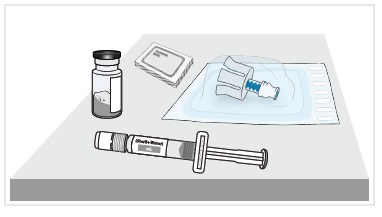
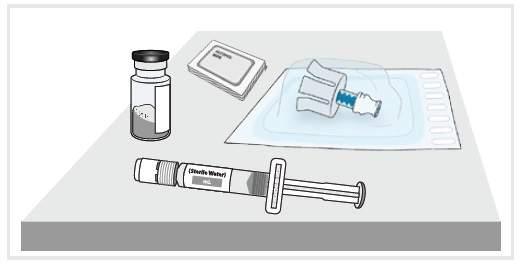
3 Remove medicine vial, prefilled syringe with sterile water for injection, alcohol wipes, and vial adapter from the kit.
4a Check medicine vial
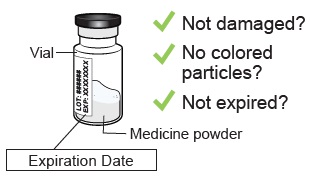
Check the vial label to confirm the medicine is not expired.
Inspect the medicine powder.
It should be white to off-white. Do not use if expired, damaged, discolored, or you can see particles in it.
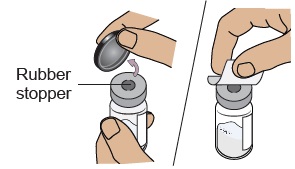
Do not use if expired, damaged, discolored, or you can see particles in it.4b Remove plastic cap and clean vial
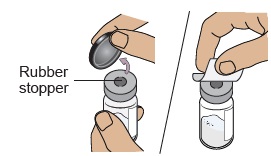
Flip off the plastic cap and clean the rubber stopper on top of the vial with an alcohol wipe. Do not use if vial cap is missing.
Do not use if vial cap is missing. Do not touch the cleaned rubber stopper.
Do not touch the cleaned rubber stopper.Set vial aside on a clean, flat surface.
5a Align vial adapter to vial
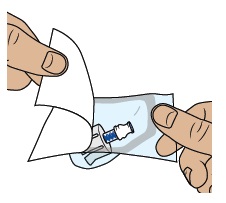
Peel open the vial adapter package and remove vial adapter from its package.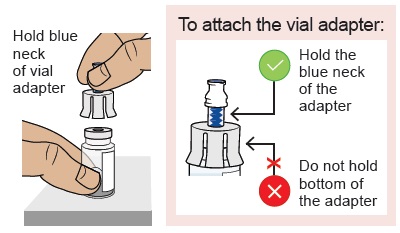
Hold the blue neck of the vial adapter and align the vial adapter on top of the vial.
 Do not touch the inside of the vial adapter.
Do not touch the inside of the vial adapter.5b Attach vial adapter to vial
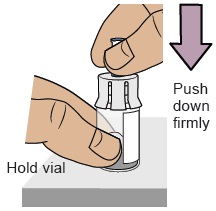
Hold the vial with one hand. Push down firmly so it snaps in place (you may feel some resistance).5c Clean vial adapter
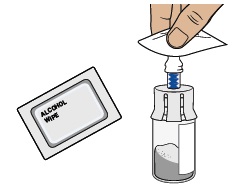
Clean the top of the vial adapter with an alcohol wipe.6 Check prefilled syringe
Confirm the sterile water inside the prefilled syringe is clear and the product is not expired or leaking.
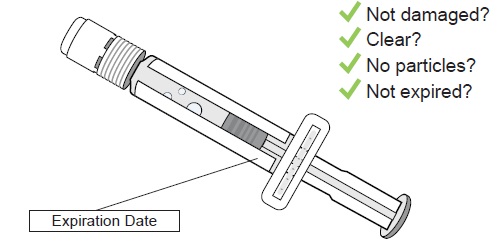
 Do not use if you see any clumps, particles, discoloration, or product is expired. You might see air bubbles. This is okay.
Do not use if you see any clumps, particles, discoloration, or product is expired. You might see air bubbles. This is okay.7 Snap off prefilled syringe white cap
Snap off the prefilled syringe cap along the perforation.
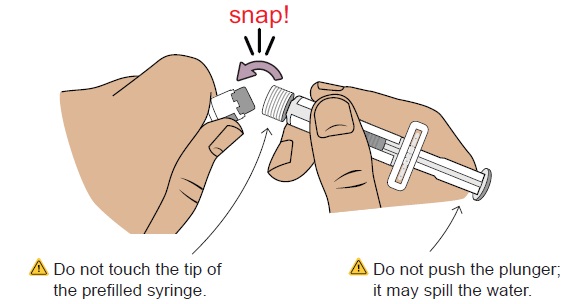
8 Connect prefilled syringe to vial adapter
Pick up the vial with the vial adapter attached.
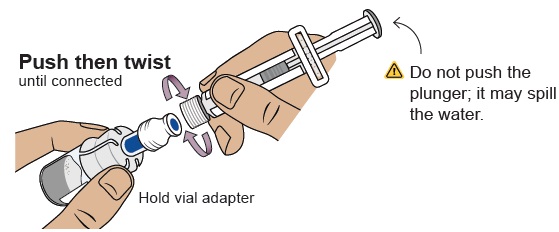
Align the prefilled syringe tip on the blue circle of the vial adapter.Push then twist the prefilled syringe onto the vial adapter until you cannot turn further. While twisting, be sure to hold onto the vial adapter.
9 Transfer sterile water from prefilled syringe to vial
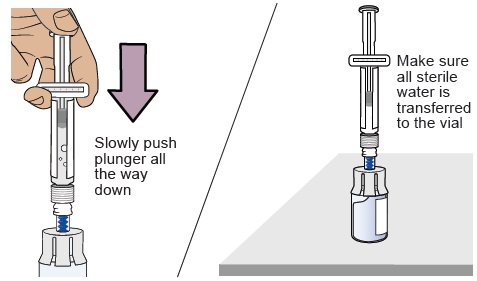
Slowly push the plunger all the way down to transfer all the sterile water into the vial (the plunger will move up; this is normal).10 Swirl to mix medicine
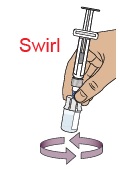
 Do not shake the vial.
Do not shake the vial.
Hold the prefilled syringe and gently swirl the vial in a circular motion until the powder is fully dissolved. This may take up to 2 minutes.
When the medicine is mixed well, it should be clear. If not, repeat this step until it is clear.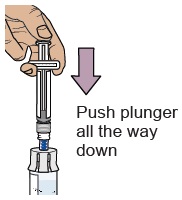
Press the plunger down to make sure all the liquid is in the vial (the plunger will move up; this is normal).11 Wait for large bubbles in the vial to go away
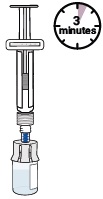
It is okay to have slight foam around edges of the vialSet vial aside for large bubbles to go away.
This may take up to 3 minutes.

12 Prepare vial by removing extra air
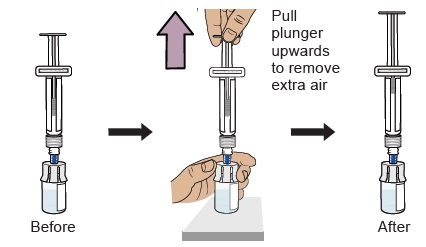
With the vial standing upright, gently pull the plunger upwards to the top of the barrel. Do not to pull the plunger out of the syringe.
Note: Pulling extra air out of the vial will help to make sure you have the right dose.13 Remove prefilled syringe from vial
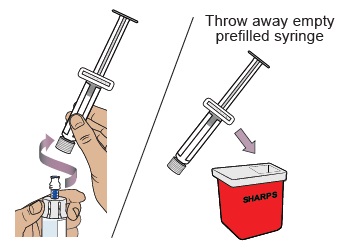
Hold the vial adapter and unscrew the prefilled syringe from the vial.
Throw away the prefilled syringe in the sharps container.
You should have a vial of medicine prepared and ready to be used in the next steps.For the next steps, you will need:
- -
- Mixed vial of medicine
- -
- Items from the Bottom Tray (needle, dosing syringe for injection, and alcohol wipes).
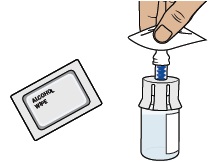
14 Clean top of vial adapter
With a new alcohol wipe from the bottom tray, clean the top of the vial adapter.
15 Remove empty dosing syringe from its package
Find the empty dosing syringe in the bottom tray and remove it from its package.
You will use this dosing syringe to measure out the medicine you need (based on your prescribed dose).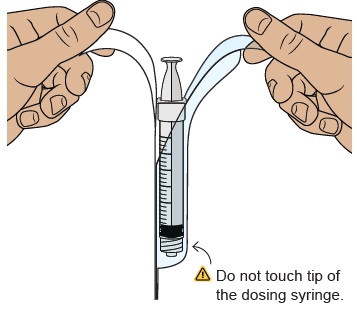
16 Pull air into the dosing syringe
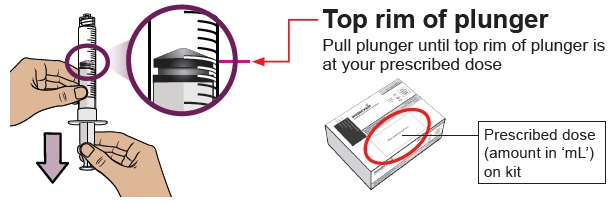
 You must do this to make sure pressure in the vial is even and you get an accurate dose.
You must do this to make sure pressure in the vial is even and you get an accurate dose.Hold the dosing syringe upright and pull down the plunger to draw air into the dosing syringe. Stop when top rim of plunger is at the amount in ‘mL’ that matches your prescribed dose. Your prescribed dose is on the prescription label on your kit.
17 Connect dosing syringe to the vial
While holding the vial adapter, push then twist the dosing syringe onto the vial adapter until it stops.
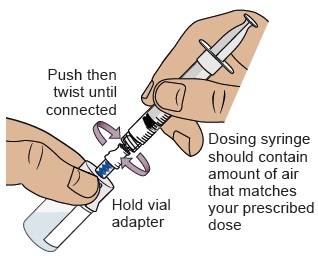
18 Push air into vial, then flip vial upside down
Push the plunger all the way down to transfer all the air into the vial.
Then hold the plunger in place with your thumb and flip the vial upside down.
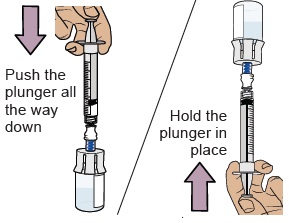
19 Pull plunger back to withdraw your prescribed dose
With the vial and dosing syringe upside down, slowly pull the plunger back.
Stop when you get to the amount in ‘mL’ that matches your prescribed dose.
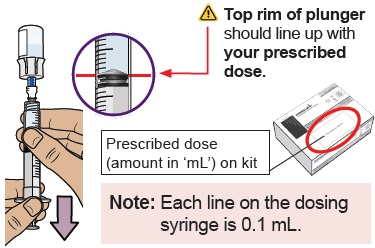
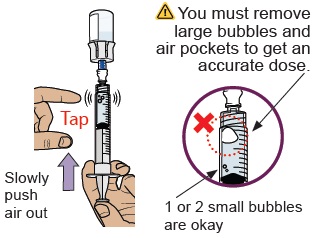
20 Check for large air bubbles and air pockets
Check to see if there are large air bubbles or an air pocket in the syringe. You will remove extra air in the next steps.
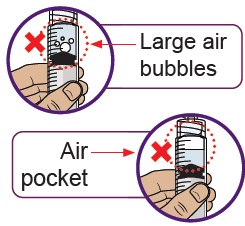
21 Remove air bubbles and air pockets from syringe
If you see large air bubbles or an air pocket, tap the side of the dosing syringe to move the air to the top.
Slowly push the plunger up to remove extra air.
22 Compare amount to prescribed dose
After removing all extra air, compare the amount in your syringe to your prescribed dose.
If you do not have your prescribed dose in your syringe, slowly pull the plunger back again to withdraw more medicine from the vial.
Repeat Steps 19 to 21 until you get your prescribed dose and no large bubbles or air pockets remain.
23 Confirm your prescribed dose
Before you continue, check to make sure you have the prescribed dose in the dosing syringe.
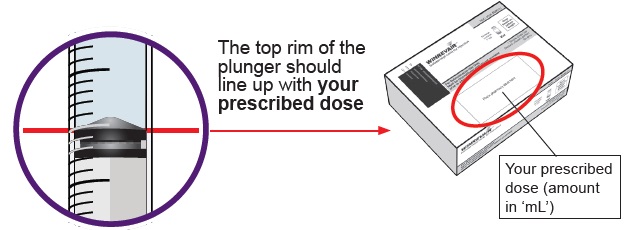
 If the amount does not match your prescribed dose, repeat Steps 19 to 22.
If the amount does not match your prescribed dose, repeat Steps 19 to 22.
24 Remove the dosing syringe from the vial and set dosing syringe aside
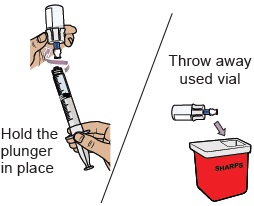
Hold the plunger in place with one hand. With the other hand, hold the vial adapter and unscrew the filled dosing syringe from the vial.
Throw away the used vial into the sharps container.
Place the filled dosing syringe on a clean, flat surface.
 Do not touch the dosing syringe tip or let it to touch any surfaces.
Do not touch the dosing syringe tip or let it to touch any surfaces.25 Attach injection needle
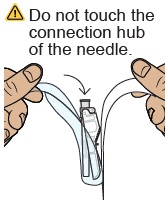
Find the needle in the bottom tray and open its package.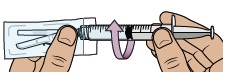
With the needle still in the package, grip the base of the needle and twist on the dosing syringe until it stops. Remove the needle package.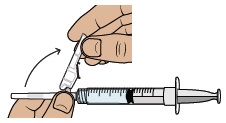
Move the safety shield away from the needle and toward the syringe. Place the dosing syringe on a clean, flat surface.
 Do not uncap the needle.
Do not uncap the needle.26 Choose and clean your injection site
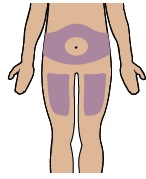
Select an injection site on your stomach (abdomen) or your upper thigh. If injecting on your stomach, avoid a 2-inch area around your belly button.
Choose a different site every time you inject.
 Do not inject into skin that is damaged, sore, tender, bruised, or has red patches.
Do not inject into skin that is damaged, sore, tender, bruised, or has red patches.
 Do not inject through clothes.
Do not inject through clothes.
Clean the injection site with a new alcohol wipe.
 Do not touch the cleaned injection site again.
Do not touch the cleaned injection site again.Now, you are ready to inject the medicine.
27 Inject your medicine
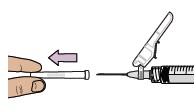
Pull the cap straight off the needle.
Throw away the cap.
 Do not touch the plunger until ready to inject so you do not lose any medicine.
Do not touch the plunger until ready to inject so you do not lose any medicine.
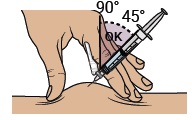
Gently pinch and hold a fold of skin where you will inject. Insert the needle with a dartlike motion at a 45° to 90° angle.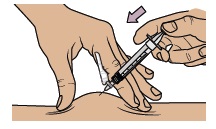
Push the plunger with slow, steady pressure all the way down until the dosing syringe is empty. Confirm all the medicine has been injected. You can let go of the skin fold now. Keep your fingers away from the needle at all times.
Keep your fingers away from the needle at all times.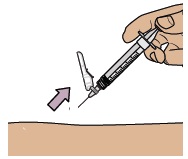
While keeping the plunger pushed in, remove the needle from your skin at the same angle you inserted it.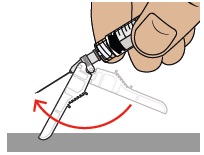
To reapply the safety shield, push the shield against a flat surface until you hear a “click” and see that the needle is covered.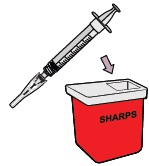
Throw away the dosing syringe and used items in a sharps disposal container.
 Do not remove the needle from the dosing syringe.
Do not remove the needle from the dosing syringe.How to throw away WINREVAIR - Throw away any used vials (including any remaining WINREVAIR liquid), needle, vial and needle caps, and used syringes in an FDA-cleared sharps disposal container.
- Do not throw away the WINREVAIR vials, syringes, or needle in your household trash.
- Do not reuse any of the supplies. WINREVAIR is for one time use only.
- Important: Always keep the sharps disposal container out of reach of children and pets.

If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharp objects being able to come out,
- upright and stable during use,
- leak resistant, and
- properly labeled to warn of hazardous waste inside the container.
When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to throw away your sharps disposal container. There may be state or local laws about how you should throw away used needles, dosing syringes, and prefilled syringes.
For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at: http://www.fda.gov/safesharpsdisposal.
 Do not recycle your used sharps container.
Do not recycle your used sharps container.What should I do if I am bleeding at the injection site?
Place a cotton ball or bandage on your skin right away and apply a small amount of pressure. If the bleeding does not stop, call your healthcare provider right away.
Where can I find my prescribed dose?
Your prescribed dose in ‘mL’ is on your prescription label on your kit. Contact your healthcare provider if you cannot find your prescribed dose.
What should I do if I accidentally get some medicine on my skin or my work surface?
Wash the area thoroughly with soap and water right away.
What should I do if I am not sure I administered my prescribed dose correctly?
Call your pharmacy or healthcare provider.
What should I do if the plunger of my dosing syringe moves automatically when I try to withdraw medicine from the vial?
Do not worry if your plunger moves slightly on its own when you are filling your dosing syringe with medicine.
With one hand, hold the plunger in place to stop the plunger from moving. With the other hand, unscrew the vial from the dosing syringe.
After unscrewed, it is safe to let go of the plunger.
You can avoid this automatic plunger movement by pushing air into the vial before filling your dosing syringe with medicine. Refer to Steps 16 to 23 for detailed instructions.
What should I do if my kit parts are damaged or are discolored, cloudy, or have particles?
Do not use your kit. Call your pharmacy to get a new kit.
What should I do if my medicine does not turn clear after mixing and swirling?
Do not use the medicine if you have swirled the medicine vial for about 2 minutes and let it stand for another 3 minutes but your medicine vial remains cloudy or has clumps, powder, or foreign particles. Call your pharmacy to get a new kit.
What should I do if the sterile water will not come out of the prefilled syringe?
Check that the vial adapter is attached to the vial securely. If not, hold the vial and press the vial adapter down firmly to make sure the vial adapter punctures the vial rubber stopper.
What should I do if I dropped my kit components?
Do not use if any items are damaged. If you are unsure, call your pharmacy to get a new kit.
Can I use my kit that has been left out of the refrigerator?
Store the injection kit in the refrigerator until ready to use it. If the unused injection kit has been out of the refrigerator up to 77°F (25°C) for more than 24 hours, please contact your pharmacy or healthcare provider.
Do I need to use mixed medicine right away?
We recommend you inject the medicine right away after mixing but no later than 4 hours after mixing. If it has been more than 4 hours, throw away unused mixed medicine. If you have questions, please contact your pharmacy or healthcare provider.
How can I get help preparing and giving my injection?
If you have questions about how to give WINREVAIR the correct way or need more information, you can call your pharmacy or healthcare provider.
Call your doctor for advice about side effects.
To report side effects or product quality complaints, please call Merck Sharp & Dohme LLC at 1-800-672-6372. You can also report side effects to FDA at 1-800-FDA-1088.
Manufactured by:
Merck Sharp & Dohme LLC
Rahway, NJ 07065, USA
U.S. License Number 0002Copyright © 2024 Merck & Co., Inc., Rahway, NJ, USA, and its affiliates.
All rights reserved.usifu1-mk7962-i-2403r000
This Instructions for Use has been approved by the U.S. Food and Drug Administration
Close
Approved: March 2024 -
INSTRUCTIONS FOR USEImportant: Read this booklet first - Important - INSTRUCTIONS FOR USE - Mix - WINREVAIR™ (WIN-reh-vair) (sotatercept-csrk) for injection, for subcutaneous use ...
Important: Read this booklet first
Important
INSTRUCTIONS FOR USE
Mix
WINREVAIR™ (WIN-reh-vair)
(sotatercept-csrk)
for injection, for subcutaneous use
How to use your WINREVAIR injection kit
Combine For package containing 2 vials
For package containing 2 vialsWithdraw
For subcutaneous injection only (inject directly under the skin)
InjectIn this booklet:
Important information for patients or caregivers
Get to know the parts of your kit
Important information you need to know before injecting WINREVAIR
 Mix powdered medicine into liquid form
Mix powdered medicine into liquid form
 Combine medicine from both vials
Combine medicine from both vials
Before you prepare and inject WINREVAIR, you must be:
- trained by a healthcare provider following this Instructions for Use booklet step-by-step, and
- capable of independent administration of WINREVAIR as determined by a healthcare provider
Before using WINREVAIR, make sure you are trained to do the following correctly:
- Reconstitute (mix) the medicine
- Combine medicine from both vials
- Measure the correct amount of medicine according to your prescription
- Select and prepare a proper injection site
- Inject the medicine subcutaneously

Read this booklet
Read this Instructions for Use from start to finish before using WINREVAIR and each time you get a refill. There may be new information.
Start with your healthcare provider
Do not use WINREVAIR until your healthcare provider has shown you or your caregiver the right way to prepare and inject it. Your healthcare provider should show you how to inject WINREVAIR before you use it for the first time.
Questions?
If you have questions about how to give WINREVAIR the right way or need more information, call your pharmacy or healthcare provider.
For general product information visit www.WINREVAIR.com or call Merck Sharp & Dohme LLC at 1-800-672-6372.How to use WINREVAIR
Before injecting WINREVAIR, make sure you read the full instructions in this booklet to get your prescribed dose.
 Mix medicine in vial 1
Mix medicine in vial 1 Mix medicine in vial 2
Mix medicine in vial 2 Combine medicine mixture from vial 1 to vial 2
Combine medicine mixture from vial 1 to vial 2 Withdraw your prescribed dose from vial 2
Withdraw your prescribed dose from vial 2 Then, inject your medicine
Then, inject your medicineProceed to the next pages for step-by-step directions.
Get to know the parts of your kit
Top Tray
Use to mix the medicine
 2 Vials of WINREVAIR medicine powder
2 Vials of WINREVAIR medicine powder
 2 Prefilled syringes with sterile water for injection to mix medicine powder into liquid form
2 Prefilled syringes with sterile water for injection to mix medicine powder into liquid form 2 Vial adapters to connect the vial and syringe
2 Vial adapters to connect the vial and syringeBottom Tray
Use to inject the medicine
Needle for injection
Empty dosing syringe to measure, withdraw, and inject your medicineImportant information you need to know before injecting WINREVAIR
- You must mix WINREVAIR before using it. Make sure the medicine powder in the vial is completely dissolved before you inject.
- Check your prescribed dose (amount in ‘mL’) each time you use WINREVAIR. Your prescribed dose may change. Your prescribed dose is on the prescription label on your kit.
- Use only the supplies that are in the kit to prepare your prescribed dose.
- Do not open the package or mix the medicine until you are ready to use it.
- Do not reuse any of the supplies. After your injection, throw away the used vials with any remaining WINREVAIR medicine, needle, caps, and syringes in a sharps container. See pages 38-39 for more information.

Store WINREVAIR injection kit in the refrigerator at 36°F to 46°F (2°C to 8°C). Do not freeze.

Store in the original carton to protect from light.

Keep injection kit out of the reach of children and pets.
Before you can prepare and inject WINREVAIR, you must first be trained and determined to be capable of independent administration of WINREVAIR by a healthcare provider. 1 Check WINREVAIR injection kit and expiration date
Remove the WINREVAIR injection kit from the refrigerator. 
 Check the expiration date and look for any signs of damage on the kit or on the supplies.
Check the expiration date and look for any signs of damage on the kit or on the supplies.
 If expired or damaged, do not use.
If expired or damaged, do not use.
Call your pharmacy to get a new kit. Check that you have the medicine that your healthcare provider prescribed.
Check that you have the medicine that your healthcare provider prescribed.2 Let your kit come to room temperature, gather supplies, and wash your hands
Wait 15 minutes to allow your kit to warm to room temperature.
Cold medicine is more painful to inject.

Along with your kit, gather these items and find a clean, flat surface where you will prepare and inject your dose.
 Sharps disposal container
Sharps disposal container Gauze, cotton ball, or bandage
Gauze, cotton ball, or bandageWash your hands with soap and water.

Mix powdered medicine into liquid form
Start with the Top Tray
3 Remove medicine vial 1, prefilled syringe with sterile water for injection 1, alcohol wipes, and vial adapter 1 from the kit.
4a Check medicine vial

Check the vial label to confirm the medicine is not expired.
Inspect the medicine powder.
It should be white to off-white. Do not use if expired, damaged, discolored, or you can see particles in it.
Do not use if expired, damaged, discolored, or you can see particles in it.4b Remove plastic cap and clean vial
Flip off the plastic cap and clean the rubber stopper on top of the vial with an alcohol wipe. Do not use if vial cap is missing.
Do not use if vial cap is missing. Do not touch the cleaned rubber stopper.
Do not touch the cleaned rubber stopper.Set vial aside on a clean, flat surface.
5a Align vial adapter to vial
Peel open the vial adapter package and remove vial adapter from its package.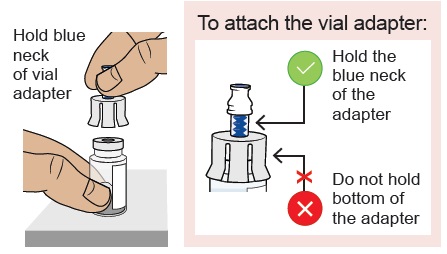
Hold the blue neck of the vial adapter and align the vial adapter on top of the vial.
 Do not touch the inside of the vial adapter.
Do not touch the inside of the vial adapter.5b Attach vial adapter to vial
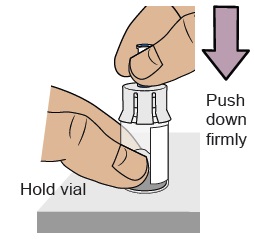
Hold the vial with one hand. Push down firmly so it snaps in place (you may feel some resistance).5c Clean vial adapter
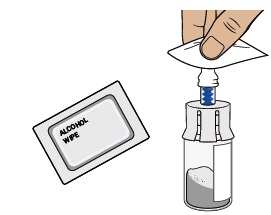
Clean the top of the vial adapter with an alcohol wipe.6 Check prefilled syringe
Confirm the sterile water inside the prefilled syringe is clear and the product is not expired or leaking.
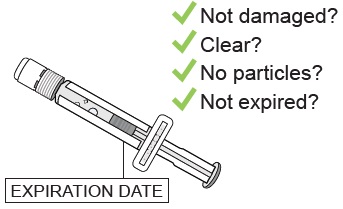
 Do not use if you see any clumps, particles, discoloration, or product is expired. You might see air bubbles. This is okay.
Do not use if you see any clumps, particles, discoloration, or product is expired. You might see air bubbles. This is okay.7 Snap off prefilled syringe white cap
Snap off the prefilled syringe cap along the perforation.
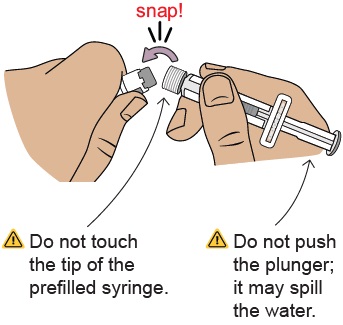
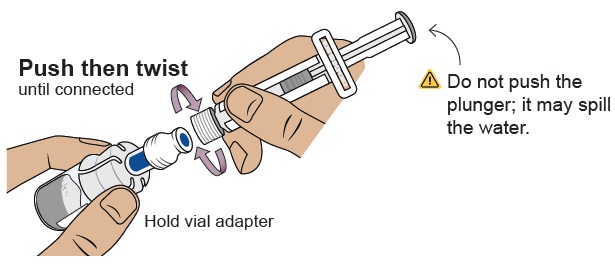
8 Connect prefilled syringe to vial adapter
Pick up the vial with the vial adapter attached.
Align the prefilled syringe tip on the blue circle of the vial adapter.
Push then twist the prefilled syringe onto the vial adapter until you cannot turn further. While twisting, be sure to hold onto the vial adapter.
9 Transfer sterile water from prefilled syringe to vial
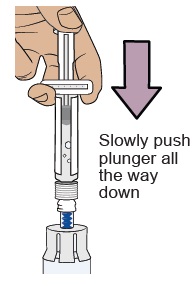
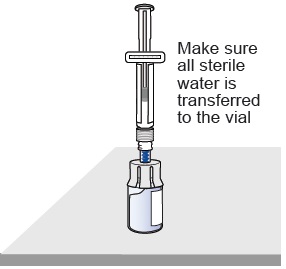
Slowly push the plunger all the way down to transfer all the sterile
water into the vial (the plunger will move up; this is normal).Leave the prefilled syringe attached to the vial and set aside on a flat surface. 10 Prepare vial 2, prefilled syringe 2, and vial adapter 2
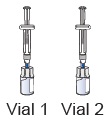
Stop and check to make sure you have both vial 1 and vial 2 prepared. You need both vials prepared before you can continue to Step 11. 11 Swirl to mix medicine
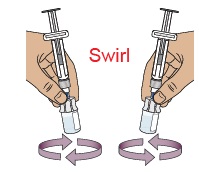
 Do not shake the vials.
Do not shake the vials.
Hold 1 prefilled syringe in each hand and gently swirl both vials in a circular motion until the powder is fully dissolved. This may take up to 2 minutes.
When the medicine is mixed well, it should be clear. If not, repeat this step until it is clear.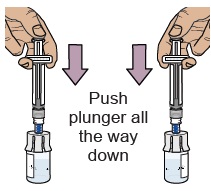
For each vial, press the plunger down to make sure all the liquid is in the vial (the plunger will move up; this is normal).12 Wait for large bubbles in the vials to go away
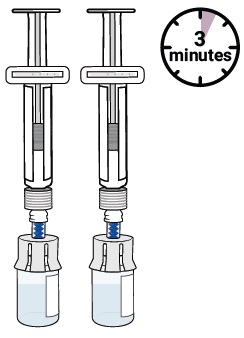
It is okay to have slight foam around edges of the vialsSet both vials aside for large bubbles to go away.
This may take up to 3 minutes.

13 Prepare vials by removing extra air
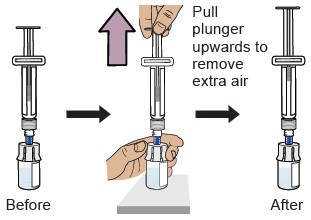
Start with either vial. With the vial standing upright, gently pull the plunger upwards to the top of the barrel. Do not pull the plunger out of the syringe.
Note: Pulling extra air out of the vials will help to make sure you have the right dose.

14 Remove prefilled syringes from vials
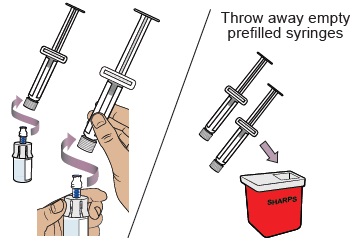
Hold the vial adapters and unscrew both prefilled syringes from the vials.
Throw away both prefilled syringes in the sharps container.
You should have 2 vials of medicine prepared and ready to be combined in the next steps.Combine medicine from both vials
For the next steps, you will need:
- -
- Mixed vial 1 and vial 2
- -
- Items from the Bottom Tray (needle, dosing syringe for injection, and alcohol wipes).
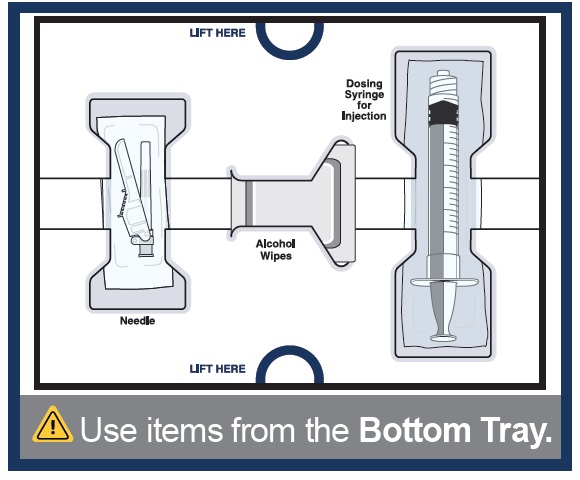
15 Clean top of both vial adapters
With 2 alcohol wipes from the bottom tray, clean the top of the vial adapters. Use a new wipe for each vial adapter.
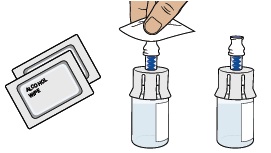
16 Remove empty dosing syringe from its package
Find the empty dosing syringe in the bottom tray and remove it from its package.
You will use this dosing syringe to combine medicine from both vials and measure out the medicine you need (based on your prescribed dose).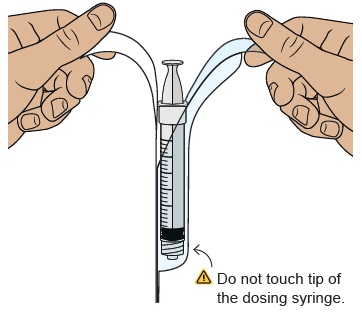
17 Pull air into the dosing syringe
 You must do this to make sure pressure in the vial is even and you get an accurate dose.
You must do this to make sure pressure in the vial is even and you get an accurate dose.Hold the dosing syringe upright and pull down the plunger to draw in 1.5 mL of air.
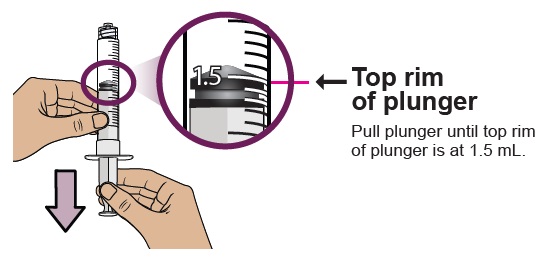
18 Connect dosing syringe to one of the vials
While holding the vial adapter, push then twist the dosing syringe onto the vial adapter until it stops.
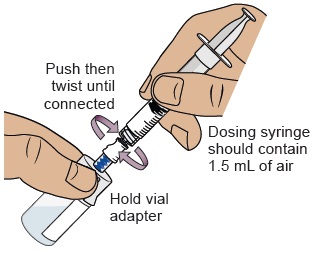
19 Push air into vial, then flip upside down
Push the plunger all the way down to transfer all the air into the vial.
Then hold the plunger in place with your thumb and flip the vial upside down.
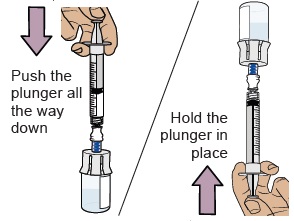
20 Withdraw all the medicine from 1st vial
With the vial and dosing syringe upside down, slowly pull the plunger back. Stop at 1.5 mL.
This will make sure all the medicine from the vial is in the dosing syringe.
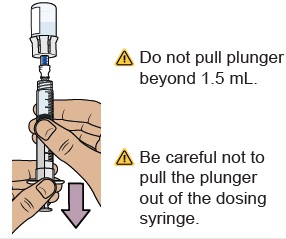
21 Remove 1st vial from the dosing syringe
Hold the vial adapter and unscrew the filled dosing syringe from the vial.
Throw away the empty vial into the sharps container.
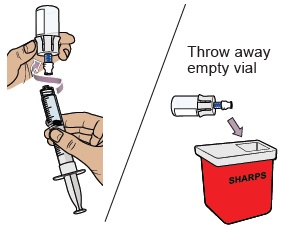
22 Connect the dosing syringe to the 2nd vial
While holding the vial adapter of the 2nd vial, push then twist the partially filled dosing syringe onto the vial adapter until it stops.
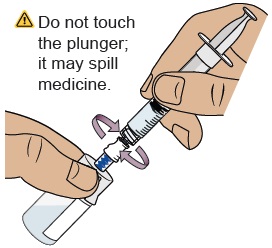
23 Push all the medicine into the 2nd vial, then flip vial upside down
Slowly push the plunger all the way down to transfer all of the medicine into the vial. This combines the medicine from both vials.
Hold the plunger in place with your thumb and flip the vial upside down.
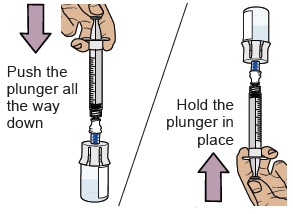
24 Pull plunger back to withdraw your prescribed dose
With the vial and dosing syringe upside down, slowly pull the plunger back.
Stop when you get to the amount in ‘mL’ that matches your prescribed dose.
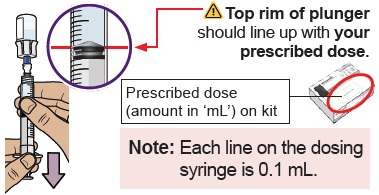
25 Check for large bubbles and air pockets
Check to see if there are large air bubbles or an air pocket in the syringe. You will remove extra air in the next steps.
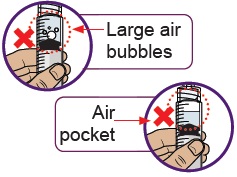
26 Remove air bubbles and air pockets from syringe
If you see large air bubbles or an air pocket, tap the side of the dosing syringe to move the air to the top.
Slowly push the plunger up to remove extra air.
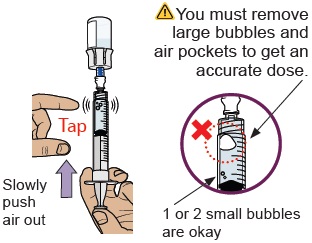
27 Compare amount to prescribed dose
After removing all extra air, compare the amount in your syringe to your prescribed dose.
If you do not have your prescribed dose in your syringe, slowly pull the plunger back again to withdraw more medicine from the vial.
Repeat Steps 24 to 26 until you get your prescribed dose and no large bubbles or air pockets are visible.
28 Confirm your prescribed dose
Before you continue, check to make sure you have the prescribed dose in the dosing syringe.
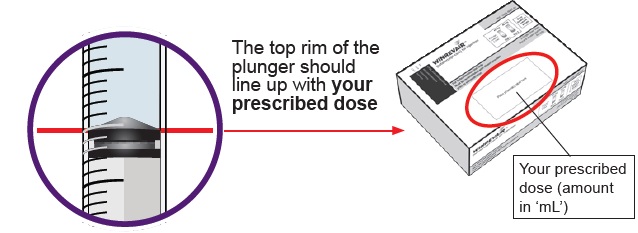
 If the amount does not match your prescribed dose, repeat Steps 24 to 27.
If the amount does not match your prescribed dose, repeat Steps 24 to 27.
29 Remove the dosing syringe from the vial and set dosing syringe aside
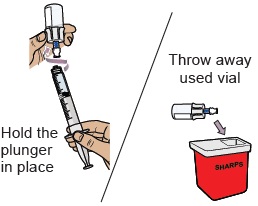
Hold the plunger in place with one hand. With the other hand, hold the vial adapter and unscrew the filled dosing syringe from the vial.
Throw away the used vial into the sharps container.
Place the filled dosing syringe on a clean, flat surface.
 Do not touch the dosing syringe tip or let it to touch any surfaces.
Do not touch the dosing syringe tip or let it to touch any surfaces.30 Attach injection needle to dosing syringe
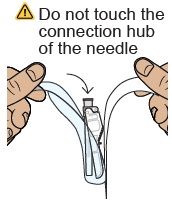
Find the needle in the bottom tray and open its package.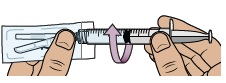
With the needle still in the package, grip the base of the needle and twist on the dosing syringe until it stops. Remove the needle package.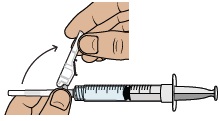
Move the safety shield away from the needle and toward the syringe. Place the dosing syringe on a clean, flat surface.
 Do not uncap the needle.
Do not uncap the needle.31 Choose and clean your injection site

Select an injection site on your stomach (abdomen) or your upper thigh. If injecting on your stomach, avoid a 2-inch area around your belly button.
Choose a different site every time you inject.
 Do not inject into skin that is damaged, sore, tender, bruised, or has red patches.
Do not inject into skin that is damaged, sore, tender, bruised, or has red patches.
 Do not inject through clothes.
Do not inject through clothes.
Clean the injection site with a new alcohol wipe.
 Do not touch the cleaned injection site again.
Do not touch the cleaned injection site again.Now, you are ready to inject the medicine.
32 Inject your medicine
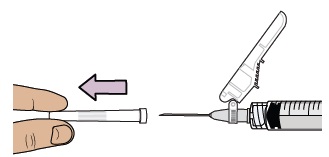
Pull the cap straight off the needle.
Throw away the cap.
 Do not touch the plunger until ready to inject so you do not lose any medicine.
Do not touch the plunger until ready to inject so you do not lose any medicine.
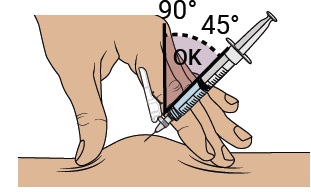
Gently pinch and hold a fold of skin where you will inject. Insert the needle with a dartlike motion at a 45° to 90° angle.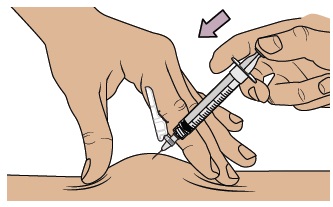
Push the plunger with slow, steady pressure all the way down until the dosing syringe is empty.
Confirm all the medicine has been injected.
You can let go of the skin fold now. Keep your fingers away from the needle at all times.
Keep your fingers away from the needle at all times.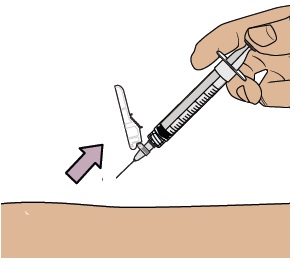
While keeping the plunger pushed in, remove the needle from your skin at the same angle you inserted it.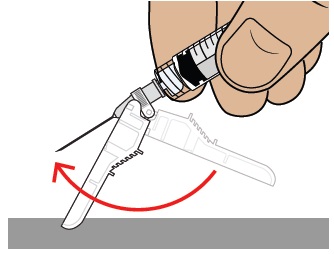
To reapply the safety shield, push the shield against a flat surface until you hear a “click” and see that the needle is covered.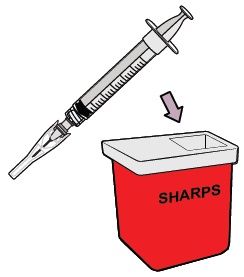
Throw away the dosing syringe and used items in a sharps container.
 Do not remove the needle from the dosing syringe.
Do not remove the needle from the dosing syringe.How to throw away WINREVAIR - Throw away any used vials (including any remaining WINREVAIR liquid), needle, vial and needle caps, and used syringes in an FDA-cleared sharps disposal container.
- Do not throw away the WINREVAIR vials, syringes, or needle in your household trash.
-
Do not reuse any of the supplies.
WINREVAIR is for one time use only. - Important: Always keep the sharps disposal container out of reach of children and pets.

If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharp objects being able to come out,
- upright and stable during use,
- leak resistant, and
- properly labeled to warn of hazardous waste inside the container.
When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to throw away your sharps container. There may be state or local laws about how you should throw away used needles, dosing syringes, and prefilled syringes.
For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at: http://www.fda.gov/safesharpsdisposal.
 Do not recycle your used sharps container.
Do not recycle your used sharps container.What should I do if I am bleeding at the injection site?
Place a cotton ball or bandage on your skin right away and apply a small amount of pressure. If the bleeding does not stop, call your healthcare provider right away.
Where can I find my prescribed dose?
Your prescribed dose in ‘mL’ is on your prescription label on your kit. Contact your healthcare provider if you cannot find your prescribed dose.
What should I do if I accidentally get some medicine on my skin or my work surface?
Wash the area thoroughly with soap and water right away.
What should I do if I am not sure I administered my prescribed dose correctly?
Call your pharmacy or healthcare provider.
What should I do if the plunger of my dosing syringe moves automatically when I try to withdraw medicine from the vial?
Do not worry if your plunger moves slightly on its own when you are filling your dosing syringe with medicine.
With one hand, hold the plunger in place to stop the plunger from moving. With the other hand, unscrew the vial from the dosing syringe.
After unscrewed, it is safe to let go of the plunger.
You can avoid this automatic plunger movement by pushing air into the vial before filling your dosing syringe with medicine. Refer to Steps 17 to 28 for detailed instructions.
What should I do if my kit parts are damaged or are discolored, cloudy, or have particles?
Do not use your kit. Call your pharmacy to get a new kit.
What should I do if my medicine does not turn clear after mixing and swirling?
Do not use the medicine if you have swirled the medicine vial for about 2 minutes and let it stand for another 3 minutes but your medicine vial remains cloudy or has clumps, powder, or foreign particles. Call your pharmacy to get a new kit.
What should I do if the sterile water will not come out of the prefilled syringe?
Check that the vial adapter is attached to the vial securely. If not, hold the vial and press the vial adapter down firmly to make sure the vial adapter punctures the vial rubber stopper.
What should I do if I dropped my kit components?
Do not use if any items are damaged. If you are unsure, call your pharmacy to get a new kit.
Can I use my kit that has been left out of the refrigerator?
Store the injection kit in the refrigerator until ready to use it. If the unused injection kit has been out of the refrigerator up to 77°F (25°C) for more than 24 hours, please contact your pharmacy or healthcare provider.
Do I need to use mixed medicine right away?
We recommend you inject the medicine right away after mixing but no later than 4 hours after mixing. If it has been more than 4 hours, throw away unused mixed medicine. If you have questions, please contact your pharmacy or healthcare provider.
How can I get help preparing and giving my injection?
If you have questions about how to give WINREVAIR the correct way or need more information, you can call your pharmacy or healthcare provider.
Call your doctor for advice about side effects.
To report side effects or product quality complaints, please call Merck Sharp & Dohme LLC at 1-800-672-6372. You can also report side effects to FDA at 1-800-FDA-1088.
Manufactured by:
Merck Sharp & Dohme LLC
Rahway, NJ 07065, USA
U.S. License Number 0002Copyright © 2024 Merck & Co., Inc., Rahway, NJ, USA, and its affiliates.
All rights reserved.usifu2-mk7962-i-2403r000
This Instructions for Use has been approved by the U.S. Food and Drug Administration
Approved: March 2024
Close -
PRINCIPAL DISPLAY PANEL - 45 mg Vial Kit CartonNDC 0006-5090-01 - WINREVAIR™ (sotatercept-csrk) for injection - 45 mg/vial - Package - contains - 1 Vial - Dose - based on - patient - weight - For Subcutaneous Use Only - Must be reconstituted with diluent ...
NDC 0006-5090-01
WINREVAIR™
(sotatercept-csrk) for injection45 mg/vial
Package
contains
1 VialDose
based on
patient
weightFor Subcutaneous Use Only
Must be reconstituted with diluent provided
prior to administration1 Single-Dose Vial
Discard unused portionRx only
Close
-
PRINCIPAL DISPLAY PANEL - 60 mg Vial Kit CartonNDC 0006-5091-01 - WINREVAIR™ (sotatercept-csrk) for injection - 60 mg/vial - Package - contains - 1 Vial - Dose - based on - patient - weight - For Subcutaneous Use Only - Must be reconstituted with diluent ...
NDC 0006-5091-01
WINREVAIR™
(sotatercept-csrk) for injection60 mg/vial
Package
contains
1 VialDose
based on
patient
weightFor Subcutaneous Use Only
Must be reconstituted with diluent provided
prior to administration1 Single-Dose Vial
Discard unused portionRx only
Close
-
PRINCIPAL DISPLAY PANEL - 45 mg 2 Vials Kit CartonNDC 0006-5087-01 - WINREVAIR™ (sotatercept-csrk) for injection - 45 mg/vial - Package - contains - 2 Vials - Dose - based on - patient - weight - For Subcutaneous Use Only - Must be reconstituted with diluent ...
NDC 0006-5087-01
WINREVAIR™
(sotatercept-csrk) for injection45 mg/vial
Package
contains
2 VialsDose
based on
patient
weightFor Subcutaneous Use Only
Must be reconstituted with diluent provided
prior to administration2 Single-Dose Vials
Discard unused portionRx only
Close
-
PRINCIPAL DISPLAY PANEL - 60 mg 2 Vials Kit CartonNDC 0006-5088-01 - WINREVAIR™ (sotatercept-csrk) for injection - 60 mg/vial - Package - contains - 2 Vials - Dose - based on - patient - weight - For Subcutaneous Use Only - Must be reconstituted with diluent ...
NDC 0006-5088-01
WINREVAIR™
(sotatercept-csrk) for injection60 mg/vial
Package
contains
2 VialsDose
based on
patient
weightFor Subcutaneous Use Only
Must be reconstituted with diluent provided
prior to administration2 Single-Dose Vials
Discard unused portionRx only
Close
-
INGREDIENTS AND APPEARANCEProduct Information
WINREVAIR sotatercept-csrk kit Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0006-5090 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0006-5090-01 1 in 1 CARTON 03/26/2024 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, SINGLE-DOSE 1 mL Part 2 1 SYRINGE 1 mL Part 1 of 2 WINREVAIR sotatercept-csrk injection, powder, lyophilized, for solution Product Information Item Code (Source) NDC:0006-5068 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SOTATERCEPT (UNII: 0QI90BTJ37) (SOTATERCEPT - UNII:0QI90BTJ37) SOTATERCEPT 45 mg in 1 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) 0.40 mg in 1 mL POLYSORBATE 80 (UNII: 6OZP39ZG8H) 0.18 mg in 1 mL SUCROSE (UNII: C151H8M554) 72 mg in 1 mL TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) 2.1 mg in 1 mL Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0006-5068-99 1 mL in 1 VIAL, SINGLE-DOSE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761363 03/26/2024 Part 2 of 2 STERILE WATER water injection, solution Product Information Item Code (Source) NDC:0006-5079 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WATER (UNII: 059QF0KO0R) (WATER - UNII:059QF0KO0R) WATER 1 mL in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0006-5079-89 1 mL in 1 SYRINGE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761363 03/26/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761363 03/26/2024 WINREVAIR sotatercept-csrk kit Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0006-5091 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0006-5091-01 1 in 1 CARTON 03/26/2024 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, SINGLE-DOSE 1 mL Part 2 1 SYRINGE 1.3 mL Part 1 of 2 WINREVAIR sotatercept-csrk injection, powder, lyophilized, for solution Product Information Item Code (Source) NDC:0006-5069 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SOTATERCEPT (UNII: 0QI90BTJ37) (SOTATERCEPT - UNII:0QI90BTJ37) SOTATERCEPT 60 mg in 1 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) 0.53 mg in 1 mL POLYSORBATE 80 (UNII: 6OZP39ZG8H) 0.24 mg in 1 mL SUCROSE (UNII: C151H8M554) 96 mg in 1 mL TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) 2.8 mg in 1 mL Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0006-5069-99 1 mL in 1 VIAL, SINGLE-DOSE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761363 03/26/2024 Part 2 of 2 STERILE WATER water injection, solution Product Information Item Code (Source) NDC:0006-5080 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WATER (UNII: 059QF0KO0R) (WATER - UNII:059QF0KO0R) WATER 1.3 mL in 1.3 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0006-5080-89 1.3 mL in 1 SYRINGE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761363 03/26/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761363 03/26/2024 WINREVAIR sotatercept-csrk kit Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0006-5087 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0006-5087-01 1 in 1 CARTON 03/26/2024 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 2 VIAL, SINGLE-DOSE 2 mL Part 2 2 SYRINGE 2 mL Part 1 of 2 WINREVAIR sotatercept-csrk injection, powder, lyophilized, for solution Product Information Item Code (Source) NDC:0006-5068 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SOTATERCEPT (UNII: 0QI90BTJ37) (SOTATERCEPT - UNII:0QI90BTJ37) SOTATERCEPT 45 mg in 1 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) 0.40 mg in 1 mL POLYSORBATE 80 (UNII: 6OZP39ZG8H) 0.18 mg in 1 mL SUCROSE (UNII: C151H8M554) 72 mg in 1 mL TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) 2.1 mg in 1 mL Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0006-5068-99 1 mL in 1 VIAL, SINGLE-DOSE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761363 03/26/2024 Part 2 of 2 STERILE WATER water injection, solution Product Information Item Code (Source) NDC:0006-5079 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WATER (UNII: 059QF0KO0R) (WATER - UNII:059QF0KO0R) WATER 1 mL in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0006-5079-89 1 mL in 1 SYRINGE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761363 03/26/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761363 03/26/2024 WINREVAIR sotatercept-csrk kit Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0006-5088 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0006-5088-01 1 in 1 CARTON 03/26/2024 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 2 VIAL, SINGLE-DOSE 2 mL Part 2 2 SYRINGE 2.6 mL Part 1 of 2 WINREVAIR sotatercept-csrk injection, powder, lyophilized, for solution Product Information Item Code (Source) NDC:0006-5069 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SOTATERCEPT (UNII: 0QI90BTJ37) (SOTATERCEPT - UNII:0QI90BTJ37) SOTATERCEPT 60 mg in 1 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) 0.53 mg in 1 mL POLYSORBATE 80 (UNII: 6OZP39ZG8H) 0.24 mg in 1 mL SUCROSE (UNII: C151H8M554) 96 mg in 1 mL TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) 2.8 mg in 1 mL Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0006-5069-99 1 mL in 1 VIAL, SINGLE-DOSE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761363 03/26/2024 Part 2 of 2 STERILE WATER water injection, solution Product Information Item Code (Source) NDC:0006-5080 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WATER (UNII: 059QF0KO0R) (WATER - UNII:059QF0KO0R) WATER 1.3 mL in 1.3 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0006-5080-89 1.3 mL in 1 SYRINGE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761363 03/26/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761363 03/26/2024
CloseLabeler - Merck Sharp & Dohme LLC (118446553)
Find additional resources
(also available in the left menu)Safety
Report Adverse Events, FDA Safety Recalls, Presence in Breast Milk
Related Resources
Medline Plus, Clinical Trials, PubMed, Biochemical Data Summary
More Info on this Drug
View Labeling Archives, RxNorm, Get Label RSS Feed, View NDC Code(s)NEW!
View Labeling Archives for this drug
WINREVAIR- sotatercept-csrk kit
Number of versions: 2
| Published Date (What is this?) | Version | Files |
|---|---|---|
| May 7, 2025 | 4 (current) | download |
| Apr 5, 2024 | 2 | download |
RxNorm
WINREVAIR- sotatercept-csrk kit
Under Review - Editing is pending for RxNorm. If in scope, these drugs will include RxNorm normal forms when editing is complete.
Get Label RSS Feed for this Drug
WINREVAIR- sotatercept-csrk kit
To receive this label RSS feed
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/labelrss.cfm?setid=21693ee2-cd23-4137-ac67-066bb3a4a9b7
To receive all DailyMed Updates for the last seven days
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/rss.cfm
What will I get with the DailyMed RSS feed?
DailyMed will deliver notification of updates and additions to Drug Label information currently shown on this site through its RSS feed.
DailyMed will deliver this notification to your desktop, Web browser, or e-mail depending on the RSS Reader you select to use. To view updated drug label links, paste the RSS feed address (URL) shown below into a RSS reader, or use a browser which supports RSS feeds, such as Safari for Mac OS X.
How to discontinue the RSS feed
If you no longer wish to have this DailyMed RSS service, simply delete the copied URL from your RSS Reader.
More about getting RSS News & Updates from DailyMedWhy is DailyMed no longer displaying pill images on the Search Results and Drug Info pages?
Due to inconsistencies between the drug labels on DailyMed and the pill images provided by RxImage, we no longer display the RxImage pill images associated with drug labels.
We anticipate reposting the images once we are able identify and filter out images that do not match the information provided in the drug labels.
NDC Codes
WINREVAIR- sotatercept-csrk kit
If this SPL contains inactivated NDCs listed by the FDA initiated compliance action, they will be specified as such.
| NDC | |
|---|---|
| 1 | 0006-5068-99 |
| 2 | 0006-5069-99 |
| 3 | 0006-5079-89 |
| 4 | 0006-5080-89 |
| 5 | 0006-5087-01 |
| 6 | 0006-5088-01 |
| 7 | 0006-5090-01 |
| 8 | 0006-5091-01 |





