Label: BIMZELX- bimekizumab injection, solution
-
NDC Code(s):
50474-780-78,
50474-780-79,
50474-781-84,
50474-781-85, view more50474-781-86, 50474-781-87, 50474-782-84, 50474-782-86, 50474-783-78
- Packager: UCB, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated January 27, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use BIMZELX safely and effectively. See full prescribing information for BIMZELX. BIMZELX® (bimekizumab-bkzx) injection, for ...These highlights do not include all the information needed to use BIMZELX safely and effectively. See full prescribing information for BIMZELX.
BIMZELX® (bimekizumab-bkzx) injection, for subcutaneous use
Initial U.S. Approval: 2023RECENT MAJOR CHANGES
INDICATIONS AND USAGE
BIMZELX is a humanized interleukin-17A and F antagonist indicated for the treatment of:
- Moderate to severe plaque psoriasis (PSO) in adults who are candidates for systemic therapy or phototherapy. (1.1)
- Adults with active psoriatic arthritis (PsA). (1.2)
- Adults with active non-radiographic axial spondyloarthritis (nr-axSpA) with objective signs of inflammation. (1.3)
- Adults with active ankylosing spondylitis (AS). (1.4)
- Adults with moderate to severe hidradenitis suppurativa (HS). (1.5)
DOSAGE AND ADMINISTRATION
- Prior to treatment: (2.1)
- Evaluate patients for tuberculosis infection.
- Test liver enzymes, alkaline phosphatase, and bilirubin.
- Complete all age-appropriate vaccinations as recommended by current immunization guidelines.
-
Plaque Psoriasis
- Administer 320 mg by subcutaneous injection at Weeks 0, 4, 8, 12, and 16, then every 8 weeks thereafter. For patients weighing 120 kg or more, consider a dose of 320 mg every 4 weeks after Week 16. (2.2)
- Psoriatic Arthritis
-
Non-Radiographic Axial Spondyloarthritis
- Administer 160 mg by subcutaneous injection every 4 weeks. (2.4)
-
Ankylosing Spondylitis
- Administer 160 mg by subcutaneous injection every 4 weeks. (2.5)
-
Hidradenitis Suppurativa
- Administer 320 mg by subcutaneous injection at Week 0, 2, 4, 6, 8, 10, 12, 14 and 16, then every 4 weeks thereafter. (2.6)
- See full prescribing information for recommendations regarding missed doses, preparation and administration instructions. (2.7, 2.8, 2.9)
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
- Suicidal Ideation and Behavior (SI/B): May increase risk of SI/B. Advise patients, their caregivers, and families to monitor for the emergence or worsening of depression, suicidal ideation, or other mood changes. If such changes occur, instruct patients to promptly seek medical attention or call the National Suicide and Crisis Lifeline at 988. Carefully weigh risks and benefits of treatment with BIMZELX in patients with a history of severe depression and/or suicidal ideation or behavior. (5.1)
- Infections: May increase risk of infection. Instruct patients to seek medical advice if signs or symptoms of clinically important infection occur. If such an infection develops, do not administer BIMZELX until the infection resolves. (5.2)
- Tuberculosis (TB): Avoid use in patients with active TB. Initiate treatment of latent TB prior to BIMZELX treatment. (5.3)
- Liver Biochemical Abnormalities: Elevated serum transaminases were reported in clinical trials. Test liver enzymes, alkaline phosphatase, and bilirubin at baseline and according to routine patient management. Permanently discontinue use of BIMZELX in patients with causally - associated combined elevations of transaminases and bilirubin. (5.4)
- Inflammatory Bowel Disease (IBD): Cases of IBD were reported in clinical trials with IL-17 inhibitors, including BIMZELX. Avoid use of BIMZELX in patients with active IBD. Monitor patients for signs and symptoms of IBD and discontinue treatment if new onset or worsening of signs and symptoms occurs. (5.5)
- Immunizations: Avoid the use of live vaccines in patients treated with BIMZELX. (5.6)
ADVERSE REACTIONS
Most common adverse reactions are:
- Psoriasis and Hidradenitis Suppurativa (incidence ≥ 1%): upper respiratory tract infections, oral candidiasis, headache, injection site reactions, tinea infections, gastroenteritis, Herpes simplex infections, acne, folliculitis, other candida infections, and fatigue. (6.1)
- Psoriatic Arthritis (incidence ≥ 2%): upper respiratory tract infections, oral candidiasis, headache, diarrhea, and urinary tract infection. (6.1)
- Non-Radiographic Axial Spondyloarthritis (incidence ≥ 2%): upper respiratory tract infections, oral candidiasis, headache, diarrhea, cough, fatigue, musculoskeletal pain, myalgia, tonsilitis, transaminase increase, and urinary tract infection. (6.1)
- Ankylosing Spondylitis (incidence ≥ 2%): upper respiratory tract infections, oral candidiasis, headache, diarrhea, injection site pain, rash and vulvovaginal mycotic infection. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact UCB, Inc. at 844-599-2273 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 11/2024
Close -
Table of ContentsTable of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Plaque Psoriasis
1.2 Psoriatic Arthritis
1.3 Non-Radiographic Axial Spondyloarthritis
1.4 Ankylosing Spondylitis
1.5 Hidradenitis Suppurativa
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Evaluations and Immunization Prior to Treatment Initiation
2.2 Recommended Dosage for Plaque Psoriasis
2.3 Recommended Dosage for Psoriatic Arthritis
2.4 Recommended Dosage for Non-Radiographic Axial Spondyloarthritis
2.5 Recommended Dosage for Ankylosing Spondylitis
2.6 Recommended Dosage for Hidradenitis Suppurativa
2.7 Missed Doses
2.8 Preparation Instructions
2.9 Administration Instructions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Suicidal Ideation and Behavior
5.2 Infections
5.3 Tuberculosis
5.4 Liver Biochemical Abnormalities
5.5 Inflammatory Bowel Disease
5.6 Immunizations
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.6 Immunogenicity
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Plaque Psoriasis
14.2 Psoriatic Arthritis
14.3 Non-Radiographic Axial Spondyloarthritis
14.4 Ankylosing Spondylitis
14.5 Hidradenitis Suppurativa
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE1.1 Plaque Psoriasis - BIMZELX is indicated for the treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy. 1.2 Psoriatic ...
1.1 Plaque Psoriasis
BIMZELX is indicated for the treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy.
1.2 Psoriatic Arthritis
BIMZELX is indicated for the treatment of adults with active psoriatic arthritis.
1.3 Non-Radiographic Axial Spondyloarthritis
BIMZELX is indicated for the treatment of adults with active non-radiographic axial spondyloarthritis with objective signs of inflammation.
1.4 Ankylosing Spondylitis
BIMZELX is indicated for the treatment of adults with active ankylosing spondylitis.
Close1.5 Hidradenitis Suppurativa
BIMZELX is indicated for the treatment of adults with moderate to severe hidradenitis suppurativa.
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Evaluations and Immunization Prior to Treatment Initiation - Evaluate patients for tuberculosis (TB) infection prior to initiating treatment with BIMZELX [see Warnings and ...
2.1 Recommended Evaluations and Immunization Prior to Treatment Initiation
- Evaluate patients for tuberculosis (TB) infection prior to initiating treatment with BIMZELX [see Warnings and Precautions (5.3)].
- Test liver enzymes, alkaline phosphatase and bilirubin prior to initiating treatment with BIMZELX [see Warnings and Precautions (5.4)].
- Complete all age-appropriate vaccinations as recommended by current immunization guidelines [see Warning and Precautions (5.6)].
2.2 Recommended Dosage for Plaque Psoriasis
The recommended dosage is 320 mg by subcutaneous injection at Weeks 0, 4, 8, 12, and 16, then every 8 weeks thereafter. For patients weighing 120 kg or more, consider a dosage of 320 mg every 4 weeks after Week 16 [see Clinical Pharmacology (12.3)].
2.3 Recommended Dosage for Psoriatic Arthritis
The recommended dosage is 160 mg by subcutaneous injection every 4 weeks.
For psoriatic arthritis patients with coexistent moderate to severe plaque psoriasis, use the dosing regimen for adult patients with plaque psoriasis [see Dosage and Administration (2.2)].
2.4 Recommended Dosage for Non-Radiographic Axial Spondyloarthritis
The recommended dosage is 160 mg by subcutaneous injection every 4 weeks.
2.5 Recommended Dosage for Ankylosing Spondylitis
The recommended dosage is 160 mg by subcutaneous injection every 4 weeks.
2.6 Recommended Dosage for Hidradenitis Suppurativa
The recommended dosage is 320 mg by subcutaneous injection at Weeks 0, 2, 4, 6, 8, 10, 12, 14, and 16, then every 4 weeks thereafter.
2.7 Missed Doses
If a dose is missed, administer the dose as soon as possible. Thereafter, resume dosing at the regularly scheduled interval.
2.8 Preparation Instructions
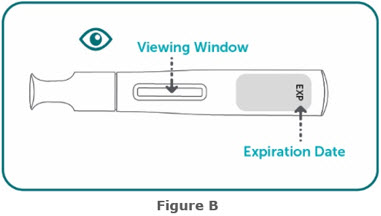
- Before injecting, remove the carton with BIMZELX from the refrigerator and allow BIMZELX to reach room temperature (30 to 45 minutes) without removing the prefilled syringes or autoinjectors from the carton to protect from light.
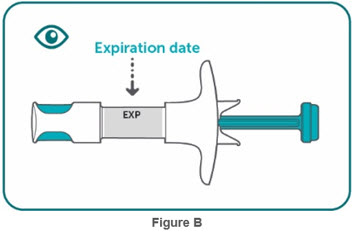
- Inspect visually for particulate matter and discoloration prior to administration, whenever solution and container permit. BIMZELX injection is clear to slightly opalescent, and colorless to pale brownish- yellow. Do not use if the solution contains visible particles, is discolored or cloudy.
Close2.9 Administration Instructions
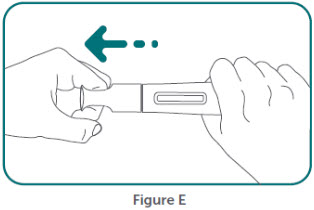
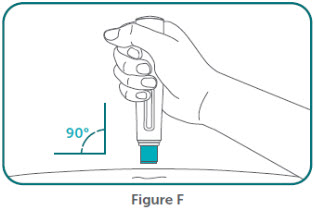
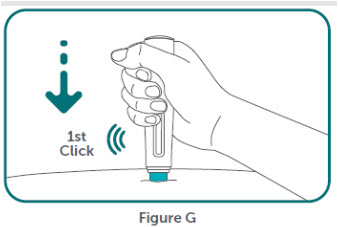
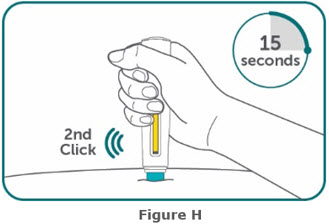
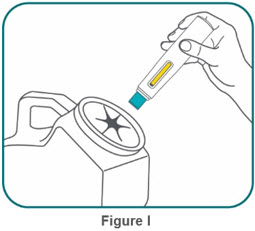
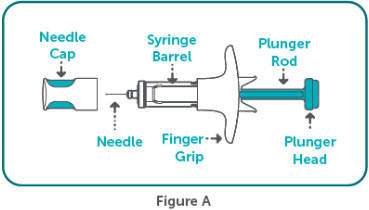
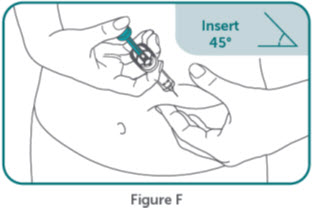
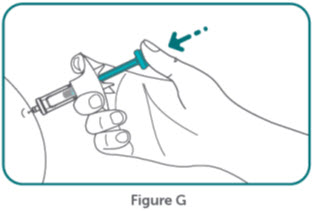
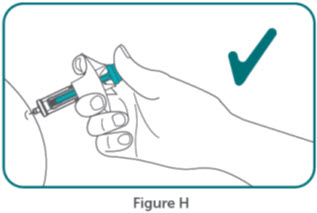
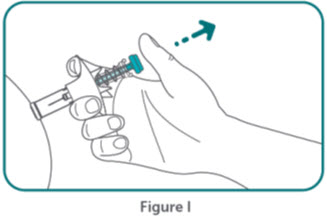
- BIMZELX is intended for use under the guidance and supervision of a healthcare professional. Patients may self-inject after training in subcutaneous injection technique. Provide proper training to patients and/or caregivers on the subcutaneous injection technique of BIMZELX according to the "Instructions for Use" [see Instructions for Use].
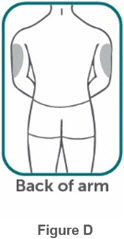
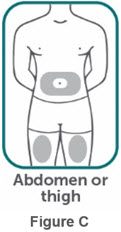
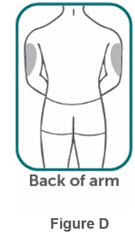
- If two separate 160 mg injections are used to achieve the recommended dose, administer each injection subcutaneously at a different anatomic location (such as thighs, abdomen or back of upper arm). Discard the syringes or autoinjectors after use. Do not reuse.
- Do not inject BIMZELX within 2 inches (5 cm) of the navel or into areas where the skin is tender, bruised, red, hard, thick, scaly, or affected by psoriasis. Administration of BIMZELX in the upper, outer arm may only be performed by a healthcare professional or caregiver. Rotate the injection site with each injection.
-
3 DOSAGE FORMS AND STRENGTHSInjection (1 mL): 160 mg/mL clear to slightly opalescent, and colorless to pale brownish-yellow solution in a single-dose prefilled syringe or single-dose prefilled autoinjector. Injection (2 ...Close
- Injection (1 mL): 160 mg/mL clear to slightly opalescent, and colorless to pale brownish-yellow solution in a single-dose prefilled syringe or single-dose prefilled autoinjector.
- Injection (2 mL): 320 mg/2 mL (160 mg/mL) clear to slightly opalescent, and colorless to pale brownish-yellow solution in a single-dose prefilled syringe or single-dose prefilled autoinjector.
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Suicidal Ideation and Behavior - An increased incidence of new onset or worsening suicidal ideation and behavior was observed in subjects treated with BIMZELX. A causal association between ...
5.1 Suicidal Ideation and Behavior
An increased incidence of new onset or worsening suicidal ideation and behavior was observed in subjects treated with BIMZELX. A causal association between treatment with BIMZELX and increased risk of suicidal ideation and behavior has not been definitively established.
Suicidal ideation and behavior were prospectively monitored using the Columbia Suicide Severity Rating Scale (C-SSRS) in clinical trials. The C-SSRS is an interview-based instrument used to monitor for the presence and severity of suicidal ideation (ranging from "none" to "active suicidal ideation with specific plan and intent") and behaviors (rating the injury and potential lethality of self-injury, if present).
Plaque Psoriasis
During the two 16-week, placebo-controlled periods of Trials Ps-1 and Ps-2, higher rates of suicidal ideation as assessed by C-SSRS were reported in BIMZELX-treated subjects than in subjects receiving placebo. Pooled analysis of C-SSRS data indicated that 12/670 (1.8%) BIMZELX-treated subjects and 1/169 (0.6%) subjects receiving placebo reported passive suicidal ideation with an estimated relative risk of 3.0 (95% confidence interval: 0.39, 22.74). Subjects without a prior history of SI/B treated with BIMZELX also reported a higher rate of new onset suicidal ideation on the C-SSRS than subjects receiving placebo (1.3% vs 0.6%). During the open-label extension trial, one completed suicide was reported in a BIMZELX-treated subject [see Adverse Reactions (6.1)].
Psoriatic Arthritis
Pooled analysis of C-SSRS data from the two 16-week, placebo-controlled periods of Trials PsA-1 and PsA-2 indicated that 2/698 (0.3%) BIMZELX-treated subjects and 3/413 (0.7%) subjects receiving placebo reported passive suicidal ideation with an estimated relative risk of 0.35 (95% confidence interval: 0.05, 2.29) [see Adverse Reactions (6.1)].
Non-Radiographic Axial Spondyloarthritis
Analysis of C-SSRS data from a 16-week, placebo-controlled period of Trial nr-axSpA-1 indicated that no subjects, being treated either with BIMZELX or placebo, reported suicidal ideation [see Adverse Reactions (6.1)].
Ankylosing Spondylitis
Analysis of C-SSRS data from a 16-week, placebo-controlled period of Trial AS-1 indicated that no subjects, being treated either with BIMZELX or placebo, reported suicidal ideation [see Adverse Reactions (6.1)].
Hidradenitis Suppurativa
During the two 16-week, placebo-controlled periods of Trials HS-1 and HS-2, higher rates of suicidal ideation as assessed by C-SSRS were reported in BIMZELX-treated subjects than in subjects receiving placebo. Based on a pooled analysis of the first 16 weeks of the placebo controlled clinical trials, 16/861 subjects in the BIMZELX group (1.9 %) reported suicidal ideation on the C-SSRS compared to 1/146 subjects in the placebo group (0.7%) with an estimated relative risk of 2.70 (95% confidence interval: 0.36, 20.12). Subjects without a prior history of SI/B treated with BIMZELX also reported a higher rate of new-onset suicidal ideation on the C-SSRS than subjects treated with placebo (0.9% vs. 0%). [see Adverse Reactions 6.1].
Consider the potential risks and benefits before prescribing BIMZELX to patients with a history of severe depression or suicidal ideation or behavior. Advise patients, their caregivers, and families to monitor for the emergence or worsening of depression, suicidal ideation, or other mood changes. If such changes occur, instruct patients to promptly seek medical attention or call the National Suicide and Crisis Lifeline at 988 [see Patient Counseling Information (17)]. Refer BIMZELX-treated patients with new or worsening symptoms of depression or suicidal ideation and/or behavior to a mental health professional, as appropriate. Re-evaluate the risks and benefits of continuing treatment with BIMZELX if such events occur.
5.2 Infections
BIMZELX may increase the risk of infections, including serious infections.
Do not initiate treatment with BIMZELX in patients with any clinically important active infection until the infection resolves or is adequately treated.
In patients with a chronic infection or a history of recurrent infection, consider the risks and benefits prior to prescribing BIMZELX. Instruct patients to seek medical advice if signs or symptoms of clinically important infection occur. If a patient develops such an infection or is not responding to standard therapy, monitor the patient closely and discontinue BIMZELX until the infection resolves.
5.3 Tuberculosis
Evaluate patients for tuberculosis (TB) infection prior to initiating treatment with BIMZELX. Avoid the use of BIMZELX in patients with active TB infection. Initiate treatment of latent TB prior to administering BIMZELX. Consider anti-TB therapy prior to initiation of BIMZELX in patients with a past history of latent or active TB in whom an adequate course of treatment cannot be confirmed. Closely monitor patients treated with BIMZELX for signs and symptoms of active TB during and after treatment.
5.4 Liver Biochemical Abnormalities
Treatment with BIMZELX was associated with increased incidence of liver enzyme elevations compared to treatment with placebo in randomized clinical trials. Liver serum transaminase elevations > 3 times the upper limit of normal were reported in subjects treated with BIMZELX [see Adverse Reactions (6.1)]. Elevated liver serum transaminases resolved after discontinuation of BIMZELX.
Test liver enzymes, alkaline phosphatase, and bilirubin at baseline, periodically during treatment with BIMZELX and according to routine patient management. If treatment-related increases in liver enzymes occur and drug-induced liver injury is suspected, interrupt BIMZELX until a diagnosis of liver injury is excluded. Permanently discontinue BIMZELX in patients with causally associated combined elevations of transaminases and bilirubin. Patients with acute liver disease or cirrhosis may be at increased risk for severe hepatic injury; avoid use of BIMZELX in these patients.
5.5 Inflammatory Bowel Disease
Cases of inflammatory bowel disease (IBD) have been reported in patients treated with IL-17 inhibitors, including BIMZELX [see Adverse Reactions (6.1)]. Avoid use of BIMZELX in patients with active IBD. During BIMZELX treatment, monitor patients for signs and symptoms of IBD and discontinue treatment if new onset or worsening of signs and symptoms occurs.
Close5.6 Immunizations
Prior to initiating therapy with BIMZELX, complete all age-appropriate vaccinations according to current immunization guidelines. Avoid the use of live vaccines in patients treated with BIMZELX. Limited data are available regarding coadministration of BIMZELX with non-live vaccines [see Clinical Pharmacology (12.2)].
-
6 ADVERSE REACTIONSThe following adverse reactions have been observed with BIMZELX and are discussed in greater detail in other sections of the labeling: Suicidal Ideation and Behavior [see Warnings and Precautions ...
The following adverse reactions have been observed with BIMZELX and are discussed in greater detail in other sections of the labeling:
- Suicidal Ideation and Behavior [see Warnings and Precautions (5.1)]
- Infections [see Warnings and Precautions (5.2)]
- Liver Biochemical Abnormalities [see Warnings and Precautions (5.4)]
- Inflammatory Bowel Disease [see Warnings and Precautions (5.5)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Plaque Psoriasis Clinical Trials
In clinical trials, a total of 1,789 subjects with plaque psoriasis were treated with BIMZELX. Of these, 1,073 subjects were exposed to BIMZELX for at least one year.
The safety of BIMZELX was evaluated in two placebo-controlled trials (Ps-1 and Ps-2), an active-controlled trial (Ps-3), and an open-label extension trial. Data from Trials Ps-1 and Ps-2 in 839 subjects (mean age 45 years, 72% male, 84% White) were pooled to evaluate the safety of BIMZELX in comparison to placebo up to 16 weeks after treatment initiation. A total of 670 subjects were treated during this initial period with BIMZELX 320 mg at Weeks 0, 4, 8, 12, and 16. Table 1 summarizes the adverse reactions that occurred at a rate of 1% or greater and at a higher rate in the BIMZELX group than the placebo group.
Table 1: Adverse Reactions Occurring in ≥1% of Subjects with Plaque Psoriasis in the BIMZELX Group and More Frequently than in the Placebo Group in Trials Ps-1 and Ps-2 BIMZELX
N=670
n (%)Placebo
N=169
n (%)- *
- Upper Respiratory Infections include nasopharyngitis, upper respiratory tract infection, pharyngitis, rhinitis, viral upper respiratory tract infection, tonsillitis, sinusitis, pharyngitis streptococcal, pharyngitis bacterial, peritonsillar abscess, viral rhinitis, and influenza
- †
- Oral Candidiasis includes oral candidiasis, oropharyngeal candidiasis, oral fungal infection, fungal pharyngitis, and oropharyngitis fungal
- ‡
- Injection Site Reactions include injection site reaction, injection site erythema, injection site pain, injection site edema, injection site bruising, and injection site swelling
- §
- Tinea Infections include tinea pedis, fungal skin infection, tinea versicolor, tinea cruris, tinea infection, body tinea, and onychomycosis
- ¶
- Gastroenteritis includes Enterovirus infection, gastroenteritis, gastroenteritis bacterial, and gastroenteritis viral
- #
- Herpes Simplex Infections include herpes simplex and oral herpes
- Þ
- Other Candida Infections include vulvovaginal candidiasis, vulvovaginal mycotic infection, skin candida, and genital candidiasis.
URI* 102 (15) 24 (14) Oral Candidiasis† 61 (9) 0 (0) Headache 22 (3) 0 (0) Injection Site Reactions‡ 19 (3) 2 (1) Tinea Infections§ 18 (3) 1 (1) Gastroenteritis¶ 12 (2) 0 (0) Herpes Simplex Infections# 9 (1) 0 (0) Acne 8 (1) 0 (0) Folliculitis 8 (1) 0 (0) Other Candida InfectionsÞ 7 (1) 1 (1) Fatigue 7 (1) 0 (0) Adverse reactions that occurred in < 1% but > 0.1% of subjects in the BIMZELX group and at a higher rate than in the placebo group through Week 16 were neutropenia, eczema, otitis externa, otitis media, and pyrexia.
The safety of BIMZELX was evaluated in another active-controlled trial (Ps-4) in 743 adult subjects who received BIMZELX 320 mg every 4 weeks or every 8 weeks through Week 48.
Specific Adverse Reactions
Suicidal Ideation and Behavior: The study populations of Trial Ps-1, Trial Ps-2, Trial Ps-3 and Trial Ps-4 excluded subjects with active suicidal ideation, suicidal ideation within the month prior to screening, a history of suicide attempt within the past 5 years prior to screening, or moderately severe to severe major depression (i.e., score of ≥15 on the screening Patient Health Questionnaire-9 (PHQ-9)).
Analysis of pooled C-SSRS data from the first 16 weeks of placebo-controlled clinical trials indicated that 12/670 (1.8%) BIMZELX-treated subjects and 1/169 (0.6%) subjects receiving placebo reported passive suicidal ideation with an estimated relative risk of 3.0 (95% confidence interval: 0.39, 22.74). Subjects without a prior history of SI/B treated with BIMZELX also reported a higher rate of new onset suicidal ideation on the C-SSRS than subjects receiving placebo (1.3% vs 0.6%).
During the course of the clinical trials for plaque psoriasis, there was 1 completed suicide in the open label extension trial after 718 days of treatment (1/2,480; 0.01/100 patient-years). The completed suicide was reported in a subject without a past reported psychiatric history. There were also 3 suicide attempts (3/2,480; 0.04/100 patient-years); 2 of these subjects had a history of prior suicide attempts.
Infections: During the placebo-controlled period of Trials Ps-1 and Ps-2, infections were reported in 36% of subjects (141.7 per 100 patient-years) treated with BIMZELX compared with 23% of subjects (84.6 per 100 patient-years) receiving placebo. Serious infections occurred in 0.3% of subjects (1.0 per 100 patient-years) treated with BIMZELX and 0% subjects receiving placebo.
The most common infections were upper respiratory tract infections and Candida infections, including oral candidiasis (oral candidiasis, oropharyngeal candidiasis, oral fungal infection, fungal pharyngitis, and oropharyngitis fungal) occurring in 9% (30.6 per 100 patient-years) of subjects treated with BIMZELX and other Candida infections (vulvovaginal candidiasis, vulvovaginal mycotic infection, skin candida, and genital candidiasis) in 1% (3.4 per 100 patient-years) of subjects treated with BIMZELX compared to 0% and 1%, respectively, of subjects receiving placebo.
During the combined initial, maintenance, and open-label extension treatment periods of trials Ps-1, Ps-2, Ps-3, and the open-label extension trial, infections were reported in 63% of subjects treated with BIMZELX (120.4 per 100 patient-years). Serious infections were reported in 1.5% of subjects treated with BIMZELX (1.6 per 100 patient-years).
Inflammatory Bowel Disease: In clinical trials in subjects with plaque psoriasis, subjects with active inflammatory bowel disease were excluded. In these trials, which included 2,480 subjects exposed to BIMZELX accounting for 5,830 patient-years, adjudicated cases of new onset of inflammatory bowel disease (including ulcerative colitis (UC), Crohn's disease (CD) and IBD-undetermined) occurred in seven subjects (0.12 per 100 patient-years); the majority of these cases were serious and resulted in discontinuation of therapy.
Liver Biochemical Abnormalities: During the placebo-controlled period of Trials Ps-1 and Ps-2, liver serum transaminase elevations (> 3 times the upper limit of normal [ULN]) occurred in 1.0% of subjects treated with BIMZELX versus 0.6% of subjects receiving placebo. The time to onset of these adverse reactions varied between 28 and 198 days after starting BIMZELX treatment. Elevated liver serum transaminases resolved during continued treatment or after discontinuation of BIMZELX.
Safety through Week 56
During the maintenance period (Week 16 through Week 52 of Trial Ps-1 and Week 56 of Trial Ps-2), adverse reactions were consistent with those observed during the initial 16 weeks of treatment with BIMZELX. During the maintenance treatment periods of Trial Ps-2 and Trial Ps-3, the rates of adverse reactions were similar between subjects treated with BIMZELX 320 mg every four week and every eight weeks, after the initial 16 weeks of treatment.
Safety through Week 128
During the open-label extension trial, including data from Week 56 through Week 128, new adverse reactions of suicide attempt and a completed suicide occurred [described above in Suicidal Ideation and Behavior].
Additional Safety Data
In an active-controlled clinical trial (Trial Ps-4), 691 subjects with plaque psoriasis were treated with BIMZELX for up to 144 weeks. Adverse reactions were consistent with those observed during the initial 16 weeks of treatment and with the overall safety profile of BIMZELX.
Psoriatic Arthritis Clinical Trials
The safety of BIMZELX was evaluated in two placebo-controlled trials (PsA-1 and PsA-2). Data from Trials PsA-1 and PsA-2 in 1,111 subjects (mean age 49 years, 47% male, 96% White) were pooled to evaluate the safety of BIMZELX in comparison to placebo up to 16 weeks after treatment initiation. A total of 698 subjects were treated during this initial period with BIMZELX 160 mg at Weeks 0, 4, 8, 12, and 16. Table 2 summarizes the adverse reactions that occurred at a rate of 2% or greater and at a higher rate in the BIMZELX group than the placebo group.
Table 2: Adverse Reactions Occurring in ≥2% of Subjects with Psoriatic Arthritis in the BIMZELX Group and More Frequently than in the Placebo Group in Trials PsA-1 and PsA-2 BIMZELX
N=698
n (%)Placebo
N=413
n (%)- *
- Upper Respiratory Tract Infections (URI) includes: nasopharyngitis, upper respiratory tract infection, pharyngitis, sinusitis, and rhinitis.
URI* 99 (14) 41 (10) Headache 25 (4) 7 (2) Diarrhea 19 (3) 8 (2) Urinary Tract Infection 14 (2) 7 (2) Oral Candidiasis 16 (2) 0 Adverse reactions that occurred in < 2% but > 1% of subjects in the BIMZELX group and at a higher rate than in the placebo group through Week 16 included neutropenia (placebo: n=0; BIMZELX: n=8 (1.1%)), stomatitis (placebo: n=0; BIMZELX: n=8 (1.1%)), bronchitis (placebo: n=1 (0.2%); BIMZELX: n=11 (1.6%)), and oropharyngeal pain (placebo: n=0; BIMZELX: n=9 (1.3%)).
Specific Adverse Reactions
Suicidal Ideation and Behavior: The neuropsychiatric inclusion/exclusion criteria in PsA trials were the same as in PSO.
Based on a pooled analysis of the first 16 weeks of the placebo controlled clinical trials, 2 of the 698 subjects in the BIMZELX group (0.3%) reported passive suicidal ideation on the C-SSRS compared to 3 of 413 subjects in the placebo group (0.7%).
During the entire clinical trial program for PsA (2,664 patient-years), there were 2 cases of suicidal ideation (2/1413; 0.08/100 patient-years) and 1 suicide attempt (1/1413; 0.04/100 patient-years); all reported in BIMZELX-treated subjects with pre-existing psychiatric conditions.
Infections: During the placebo-controlled period of Trials PsA-1 and PsA-2, infections were reported in 27% of subjects (100.7 per 100 patient-years) treated with BIMZELX compared with 18% of subjects (62.8 per 100 patient-years) treated with placebo. Serious infections occurred in 0.4% of subjects (1.4 per 100 patient-years) treated with BIMZELX and 0% treated with placebo.
The most common infections were upper respiratory tract infections, nasopharyngitis, urinary tract infection and Candida infections, including oral candidiasis (oral candidiasis, oral fungal infection, and tongue fungal infection), occurring in 3.2% (10.2 per 100 patient-years) of subjects treated with BIMZELX and other Candida infections (skin candida, vulvovaginal candidiasis, and vulvovaginal mycotic infection) in 0.6% (1.8 per 100 patient-years) of subjects treated with BIMZELX compared to 0% and 1%, respectively, of subjects treated with placebo.
During the combined placebo-controlled, maintenance and open-label extension treatment periods of Trials PsA-1 and PsA-2, infections were reported in 58% of subjects treated with BIMZELX (58.5 per 100 patients-years). Serious infections were reported in 2% of subjects treated with BIMZELX (1.3 per 100 patient-years).
Inflammatory Bowel Disease: In clinical trials in subjects with psoriatic arthritis, subjects with active inflammatory bowel disease were excluded. In these trials, which included 1,413 subjects exposed to BIMZELX accounting for 2,664 patient-years, adjudicated cases of new onset of inflammatory bowel disease (including ulcerative colitis (UC) and IBD) occurred in 2 subjects (0.08 per 100 patient-years); one of these cases was serious and none resulted in discontinuation of therapy.
Liver Biochemical Abnormalities: During the placebo-controlled period of Trials PsA-1 and PsA-2, liver serum transaminase elevations (> 3 times the upper limit of normal [ULN]) occurred in 1.3% of subjects treated with BIMZELX versus 0% of subjects receiving placebo. Elevated liver serum transaminases resolved during continued treatment or after discontinuation of BIMZELX.
Non-Radiographic Axial Spondyloarthritis Clinical Trials
BIMZELX was evaluated in a placebo-controlled trial (Trial nr-axSpA-1) in subjects with non-radiographic axial spondyloarthritis (128 subjects on BIMZELX and 126 subjects on placebo). The safety profile observed in subjects with non-radiographic axial spondyloarthritis treated with BIMZELX was overall similar to the safety profile seen in subjects with psoriatic arthritis, except for cough, musculoskeletal pain, myalgia, tonsilitis, transaminase increase (placebo: n=0; BIMZELX: n=3 (2.3%) for each), and fatigue (placebo: n=1 (0.8%); BIMZELX: n=3 (2.3%)).
Specific Adverse Reactions
Suicidal Ideation and Behavior: The neuropsychiatric inclusion/exclusion criteria were the same in non-radiographic axial spondyloarthritis trials as in PSO.
During the first 16 weeks of the placebo controlled clinical trial (Trial nr-axSpA-1), no subjects in the BIMZELX or placebo group reported suicidal ideation on the C-SSRS. During the entire clinical trial program for nr-axSpA (398 patient-years), there were no cases of suicidal ideations. One suicide attempt (1/244; 0.25/100 patient-years) was reported in a BIMZELX-treated patient with pre-existing psychiatric conditions and recent life stressors.
Infections: During the placebo-controlled period of Trial nr-axSpA-1, infections were reported in 36% of subjects (144.8 per 100 patient-years) treated with BIMZELX compared with 25% of subjects (94.4 per 100 patient-years) receiving placebo. There were no reports of serious infections reported during the placebo-controlled period of the trial.
During the combined 52 week treatment period of Trial nr-axSpA-1, and subsequent open-label treatment, infections were reported in 68% of subjects treated with BIMZELX (78.0 per 100 patient-years). Serious infections were reported in 1.6% of subjects treated with BIMZELX (1.0 per 100 patient-years).
Inflammatory Bowel Disease: In the clinical trial in subjects with non-radiographic axial spondyloarthritis, subjects with active inflammatory bowel disease were excluded. In placebo-controlled, maintenance, and open label treatment periods of this trial, which included 244 subjects exposed to BIMZELX accounting for 397 patient-years, adjudicated cases of new onset of inflammatory bowel disease occurred in 1 subject (Ulcerative Colitis; 0.26 per 100 patient-years); this case of ulcerative colitis was nonserious and did not result in discontinuation of therapy.
Liver Biochemical Abnormalities: During the placebo-controlled period of Trials nr-axSpA-1, liver serum transaminase elevations (> 3 times the upper limit of normal [ULN]) occurred in 1.6% of subjects treated with BIMZELX versus 0.8% of subjects receiving placebo. Elevated liver serum transaminases resolved during continued treatment or after discontinuation of BIMZELX.
Ankylosing Spondylitis Clinical Trials
BIMZELX was evaluated in a placebo-controlled trial (Trial AS-1) in subjects with ankylosing spondylitis (221 subjects on BIMZELX and 111 subjects on placebo). The safety profile observed in subjects with ankylosing spondylitis treated with BIMZELX was overall similar to the safety profile seen in subjects with psoriatic arthritis, except for injection site pain, rash (placebo: n=1 (0.9%); BIMZELX: n=6 (2.7%), for each) and vulvovaginal mycotic infection (placebo: n=0; BIMZELX: n=5 (2.3%)).
Specific Adverse Reactions
Suicidal Ideation and Behavior: The neuropsychiatric inclusion/exclusion criteria were the same in AS trials as in PSO.
During the first 16 weeks of the placebo controlled clinical Trial AS-1, no subjects in the BIMZELX or placebo group reported suicidal ideation on the C-SSRS. During the entire clinical trial program for AS (1,844 patient-years), there was 1 case of suicidal ideation (1/684; 0.05/100 patient-years) reported in a subject with pre-existing psychiatric conditions.
Infections: During the placebo-controlled period of Trial AS-1, infections were reported in 28% of subjects (110.3 per 100 patient-years) treated with BIMZELX compared with 23% of subjects (83.7 per 100 patient-years) receiving placebo. Serious infections occurred in 1 (0.5%) subject (1.5 per 100 patient-years) treated with BIMZELX and 1 (0.9%) subject (2.9 per 100 patient-years) receiving placebo.
During the combined 52 week treatment period of Trial AS-1, and subsequent open-label treatment, infections were reported in 62% of subjects treated with BIMZELX (58.8 per 100 patient-years). Serious infections were reported in 2.7% of subjects treated with BIMZELX (1.5 per 100 patient-years).
Inflammatory Bowel Disease: In clinical trials in subjects with AS, subjects with active inflammatory bowel disease were excluded. In these phase 2/3 trials, which included 593 subjects exposed to BIMZELX accounting for 1,599 patient-years, adjudicated cases of new onset of inflammatory bowel disease (including ulcerative colitis (UC), Crohn's disease (CD) and IBD-undetermined) occurred in 6 subjects (0.38 per 100 patient-years); 4 cases were serious, and 3 cases resulted in discontinuation of therapy.
Liver Biochemical Abnormalities: During the placebo-controlled period of Trial AS-1, liver serum transaminase elevations (> 3 times the upper limit of normal [ULN]) occurred in 1.4% of subjects treated with BIMZELX versus 1.8% of subjects receiving placebo. Elevated liver serum transaminases resolved during continued treatment or after discontinuation of BIMZELX.
Hidradenitis Suppurativa Clinical Trials
BIMZELX was evaluated in two placebo-controlled trials (Trial HS-1 and Trial HS-2) in 1,007 adult subjects with moderate to severe hidradenitis suppurativa (861 BIMZELX-treated subjects and 146 subjects receiving placebo) [see Clinical Studies (14.5)]. The safety profile observed in subjects with hidradenitis suppurativa treated with BIMZELX was overall similar to the safety profile seen in subjects with PSO treated with BIMZELX.
Upon completion of both trials, a total of 657 subjects enrolled in a long-term extension treatment period for up to an additional 188 weeks.
Specific Adverse Reactions
Suicidal Ideation and Behavior: The neuropsychiatric inclusion/exclusion criteria were the same in HS trials as in PSO.
Analysis of pooled C-SSRS data from the first 16 weeks of the placebo-controlled clinical trials indicated that 16/861 (1.9%) BIMZELX-treated subjects and 1/146 (0.7%) subjects receiving placebo reported suicidal ideation with an estimated relative risk of 2.70 (95% confidence interval: 0.36, 20.12). Subjects without a prior history of SI/B treated with BIMZELX also reported a higher rate of new-onset suicidal ideation on the C-SSRS than subjects treated with placebo (0.9% vs. 0%).
There were 2 reported cases of suicidal ideation that were adjudicated as suicide attempts (2/1,041; 0.15/100 patient-years). Both subjects had a history of neuropsychiatric disorders.
Infections: During the placebo-controlled period of Trials HS-1 and HS-2, infections were reported in 33% of subjects (132.2 per 100 patient-years) treated with BIMZELX compared with 21% of subjects (76.4 per 100 patient-years) receiving placebo. Serious infections occurred in 0.1% of subjects (0.4 per 100 patient-years) treated with BIMZELX and 0% of subjects receiving placebo.
The most commonly reported infections were comparable to those reported in subjects with PSO. Oral candidiasis occurred in 7.8% of subjects (26.7 per 100 patient years) treated with BIMZELX 320 mg Q2W, 3.9% of subjects (12.9 per 100 patient-years) treated with BIMZELX 320 mg Q4W, and 0 subjects receiving placebo. Other candida infections occurred in 3.6% of subjects (12.2 per 100 patient-years) treated with BIMZELX 320 mg Q2W, 6.7% of subjects (22.6 per 100 patient-years) treated with BIMZELX 320 mg Q4W, and 0 subjects receiving placebo. Tinea infections occurred in 3.1% of subjects (10.4 per 100 patient-years) treated with BIMZELX 320 mg Q2W, 2.5% of subjects (8.1 per 100 patient-years) treated with BIMZELX 320 mg Q4W, and 0.7% (2.3 per 100 patient-years) receiving placebo.
During the combined placebo-controlled, maintenance treatment periods and open-label extension treatment periods of Trials HS-1 and HS-2, infections were reported in 68% of subjects treated with BIMZELX (104.7 per 100 patient-years). Serious infections were reported in 2.1% of subjects treated with BIMZELX (1.7 per 100 patient-years).
Inflammatory Bowel Disease: In clinical trials in subjects with hidradenitis suppurativa, subjects with active inflammatory bowel disease were excluded. During the combined placebo-controlled, maintenance, and open-label extension treatment periods of Trials HS-1 and HS-2, which included 995 subjects exposed to BIMZELX accounting for 1,272 patient-years, adjudicated cases of new onset of inflammatory bowel disease (including ulcerative colitis (UC), Crohn's disease (CD) and undetermined) occurred in 5 subjects (0.39 per 100 patient-years); 3 of these cases were serious, all of which resulted in discontinuation of therapy. Additionally, flares of pre-existing IBD occurred in 2 subjects, which resulted in discontinuation of BIMZELX in both.
Liver Biochemical Abnormalities: During the placebo-controlled period of Trials HS-1 and HS-2, liver serum transaminase elevations (> 3 times the upper limit of normal [ULN]) occurred in 1.2% of subjects treated with BIMZELX versus 0% of subjects receiving placebo. Elevated liver serum transaminases resolved during continued treatment or after discontinuation of BIMZELX.
Close6.2 Postmarketing Experience
The following adverse reactions have been reported during post-approval use of BIMZELX. Because they are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Infections: conjunctivitis, esophageal candidiasis
-
7 DRUG INTERACTIONSCYP450 Substrates - The formation of CYP450 enzymes can be altered by increased levels of certain cytokines (e.g., IL-1, IL-6, IL-10, TNFα, IFN) during chronic inflammation. Treatment with ...Close
CYP450 Substrates
The formation of CYP450 enzymes can be altered by increased levels of certain cytokines (e.g., IL-1, IL-6, IL-10, TNFα, IFN) during chronic inflammation. Treatment with BIMZELX may modulate serum levels of some cytokines.
Therefore, upon initiation or discontinuation of BIMZELX in patients who are receiving concomitant drugs which are CYP450 substrates, particularly those with a narrow therapeutic index, consider monitoring for effect (e.g., for warfarin) or drug concentration (e.g., for cyclosporine) and consider dosage modification of the CYP450 substrate.
Population pharmacokinetic (PK) data analyses indicated that the clearance of BIMZELX was not impacted by concomitant administration of cDMARDs including methotrexate, or by prior exposure to biologics.
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Pregnancy Exposure Registry - There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to BIMZELX during pregnancy. For more information ...
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to BIMZELX during pregnancy. For more information, healthcare providers or patients can contact the Organization of Teratology Information Specialists (OTIS) AutoImmune Diseases Study at 1-877-311-8972 or visit http://mothertobaby.org/pregnancy-studies/.
Risk Summary
Available data from case reports on BIMZELX use in pregnant women are insufficient to evaluate for a drug associated risk of major birth defects, miscarriage, or other adverse maternal or fetal outcomes. Transport of human IgG antibody across the placenta increases as pregnancy progresses and peaks during the third trimester; therefore, BIMZELX may be transmitted from the mother to the developing fetus (see Clinical Considerations). In an enhanced pre- and postnatal development study, no adverse developmental effects were observed in infants born to pregnant monkeys after subcutaneous administration of bimekizumab-bkzx during the period of organogenesis through parturition at doses up to 38 times the maximum recommended human dose (MRHD) (see Data).
All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. The background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Fetal/Neonatal Adverse Reactions: Because bimekizumab-bkzx may interfere with immune response to infections, risks and benefits should be considered prior to administering live vaccines to infants exposed to BIMZELX in utero. There are no data regarding infant serum levels of bimekizumab-bkzx at birth and the duration of persistence of bimekizumab-bkzx in infant serum after birth. Although a specific timeframe to delay live virus immunizations in infants exposed in utero is unknown, a minimum of 4 months after birth may be considered because of the half-life of the product.
Data
Animal Data: An enhanced pre- and postnatal developmental toxicity study was conducted in cynomolgus monkeys. Pregnant cynomolgus monkeys were administered subcutaneous doses of bimekizumab-bkzx of 20 or 50 mg/kg/week from gestation day 20 to parturition and the cynomolgus monkeys (mother and infants) were monitored for 6 months after delivery. No maternal toxicity was noted in this study. There were no treatment-related effects on growth and development, malformations, developmental immunotoxicology or neurobehavioral development. The no observed adverse effect level (NOAEL) for both maternal and developmental toxicity was identified as 50 mg/kg/week (38 times the MRHD, based on mg/kg comparison of 1.33 mg/kg/week administered as a 320 mg dose to a 60 kg individual once every 4 weeks).
8.2 Lactation
Risk Summary
There are no data on the presence of bimekizumab-bkzx in human or animal milk, the effects on the breastfed infant, or the effects on milk production. Endogenous IgG and monoclonal antibodies are transferred in human milk. The effects of local gastrointestinal exposure and limited systemic exposure in the breastfed infant to bimekizumab-bkzx are unknown. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for BIMZELX and any potential adverse effects on the breastfed infant from BIMZELX or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of BIMZELX in pediatric patients have not been established.
Close8.5 Geriatric Use
Of the 1,789 subjects with plaque psoriasis that were exposed to BIMZELX, a total of 153 subjects were 65 years of age or older, and 18 subjects were 75 years of age or older. Although no differences in safety or effectiveness were observed between subjects 65 years of age or older and younger adult subjects, clinical trials in PSO did not include sufficient numbers of subjects aged 65 years and over to determine whether they respond differently from younger adult subjects.
Of the 1,197 subjects with PsA that were exposed to BIMZELX, a total of 148 were 65 years of age and older. Although no differences in safety or effectiveness were observed between subjects 65 years of age or older and younger adult subjects, clinical trials in PsA did not include sufficient numbers of subjects aged 65 years and over to determine whether they respond differently from younger adult subjects.
Of the 244 subjects with nr-axSpA that were exposed to BIMZELX, a total of 6 were 65 years of age and older. Although no differences in safety or effectiveness were observed between subjects 65 years of age or older and younger adult subjects, the clinical trial in nr-axSpA did not include sufficient numbers of subjects aged 65 years and over to determine whether they respond differently from younger adult subjects.
Of the 330 subjects with AS that were exposed to BIMZELX, a total of 11 were 65 years of age and older. Although no differences in safety or effectiveness were observed between subjects 65 years of age or older and younger adult subjects, the clinical trial in AS did not include sufficient numbers of subjects aged 65 years and over to determine whether they respond differently from younger adult subjects.
Of the 995 subjects with hidradenitis suppurativa that were exposed to BIMZELX, a total of 18 were 65 years of age and older. Although no differences in safety or effectiveness were observed between subjects 65 years of age or older and younger adult subjects, clinical trials in HS did not include sufficient numbers of subjects aged 65 years and over to determine whether they respond differently from younger adult subjects.
-
11 DESCRIPTIONBimekizumab-bkzx, an interleukin-17 A and F antagonist, is a recombinant humanized immunoglobulin G1 (IgG1) monoclonal antibody. Bimekizumab-bkzx is produced by recombinant DNA technology in ...
Bimekizumab-bkzx, an interleukin-17 A and F antagonist, is a recombinant humanized immunoglobulin G1 (IgG1) monoclonal antibody. Bimekizumab-bkzx is produced by recombinant DNA technology in Chinese Hamster Ovary cells, and has an approximate molecular weight of 150 kDa.
BIMZELX (bimekizumab-bkzx) injection is a sterile, preservative-free, clear to slightly opalescent, and colorless to pale brownish-yellow solution for subcutaneous use.
Each BIMZELX 1 mL (160 mg/mL) prefilled syringe or prefilled autoinjector delivers 1 mL containing 160 mg bimekizumab-bkzx, glacial acetic acid (1.23 mg), glycine (16.5 mg), polysorbate 80 (0.4 mg), sodium acetate (2.83 mg), and Water for Injection, USP at pH 5.1.
Each BIMZELX 2 mL (160 mg/mL) prefilled syringe or prefilled autoinjector delivers 2 mL containing 320 mg bimekizumab-bkzx, glacial acetic acid (2.46 mg), glycine (33.0 mg), polysorbate 80 (0.8 mg), sodium acetate (5.65 mg), and Water for Injection, USP at pH 5.1.
Close -
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Bimekizumab-bkzx is a humanized immunoglobulin IgG1/ κ monoclonal antibody with two identical antigen binding regions that selectively bind to human interleukin 17A ...
12.1 Mechanism of Action
Bimekizumab-bkzx is a humanized immunoglobulin IgG1/ κ monoclonal antibody with two identical antigen binding regions that selectively bind to human interleukin 17A (IL-17A), interleukin 17F (IL-17F), and interleukin 17-AF cytokines, and inhibits their interaction with the IL-17 receptor complex. IL-17A and IL-17F are naturally occurring cytokines that are involved in normal inflammatory and immune responses. Bimekizumab-bkzx inhibits the release of proinflammatory cytokines and chemokines.
12.2 Pharmacodynamics
Elevated levels of IL-17A and IL-17F are found in lesional psoriatic skin, and lesional skin in HS. Bimekizumab-bkzx exposure-response relationships to serum biomarkers, including IL-17A and IL-17F, and the time course of such pharmacodynamic responses are unknown.
Immune Response to Inactivated or Non-Live Vaccines
Healthy individuals who received a single 320 mg dose of BIMZELX two weeks prior to vaccination with an inactivated seasonal influenza vaccine had similar antibody responses compared to individuals who did not receive BIMZELX prior to vaccination. The effectiveness of inactivated seasonal influenza vaccines and other inactivated and non-live vaccines has not been evaluated in patients treated with BIMZELX.
12.3 Pharmacokinetics
Bimekizumab-bkzx pharmacokinetics are comparable in adult patients with moderate to severe plaque psoriasis, psoriatic arthritis, non-radiographic axial spondyloarthritis, and ankylosing spondylitis.
The median peak plasma concentration of bimekizumab-bkzx was 25 (range: 12-50) μg/mL and was reached in 3-4 days. Bimekizumab-bkzx exhibited dose-proportional pharmacokinetics in patients with plaque psoriasis over a dose range of 64 mg to 480 mg (0.2 to 1.5 times the approved recommended dosage) following subcutaneous administration.
The median steady-state trough concentration of bimekizumab-bkzx was approximately 40% lower in HS subjects than that of PSO subjects.
Distribution
The median volume of distribution at steady state was 11.2 L in subjects with moderate to severe plaque psoriasis. The volume of distribution in subjects with moderate to severe hidradenitis suppurativa was estimated to be approximately 18% higher than in subjects with moderate to severe plaque psoriasis.
Elimination
The median (coefficient of variation %) clearance (CL/F) of bimekizumab-bkzx was 0.337 L/day (32.7%). The mean terminal elimination half-life was 23 days, with clearance independent of dose. The apparent clearance in subjects with moderate to severe hidradenitis suppurativa was estimated to be approximately 31% higher than in subjects with moderate to severe plaque psoriasis.
Specific Populations
No significant differences in the pharmacokinetics of bimekizumab-bkzx were observed based on age (≥18 years).
Body Weight: The average plasma concentration in adult subjects weighing ≥120 kg was predicted to be at least 30% lower than those weighing < 120 kg (median of 87 kg) [see Dosage and Administration (2.2)].
Close12.6 Immunogenicity
The observed incidence of anti-drug antibodies (ADA) is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of ADA in the studies described below with the incidence of ADA in other studies, including those of BIMZELX or of other bimekizumab products.
Across the pivotal trials in all indications, there was no identified clinically significant effect of anti-bimekizumab-bkzx antibodies, including neutralizing anti-drug antibodies, on safety or effectiveness of BIMZELX.
Plaque Psoriasis
During the 52–56-week treatment period in Trial Ps-1, Trial Ps-2, and Trial Ps-3 [see Clinical Studies (14.1)], 116/257 (45%) of BIMZELX-treated subjects (at the recommended dosage) developed anti-bimekizumab-bkzx antibodies (also referred to as ADA). Of the BIMZELX-treated subjects who developed ADA in these trials, approximately 16% had neutralizing antibodies.
Psoriatic Arthritis
During the 52-week treatment period in Trial PsA-1 [see Clinical Studies (14.2)], 201/431 (47%) of subjects treated with BIMZELX had ADA, and 18% had neutralizing ADA.
Non-Radiographic Axial Spondyloarthritis
During the 52-week treatment period in Trial nr-axSpA-1 [see Clinical Studies (14.3)], 68/119 (57%) of BIMZELX-treated subjects had anti-bimekizumab-bkzx ADA, and approximately 25% had neutralizing ADA.
Ankylosing Spondylitis
During the 52-week treatment period in Trial AS-1 [see Clinical Studies (14.4)], 86/194 (44%) of BIMZELX-treated subjects had anti-bimekizumab-bkzx ADA, and approximately 20% had neutralizing ADA.
Hidradenitis Suppurativa
During the 48-week treatment period in Trials HS-1 and HS-2 [see Clinical Studies (14.5)], 171/291 (59%) of BIMZELX-treated subjects had anti-bimekizumab-bkzx ADA, and of those who developed ADA, approximately 63% had neutralizing ADA.
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenicity and mutagenicity studies have not been conducted with bimekizumab-bkzx. No effects on fertility parameters such as ...Close
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity and mutagenicity studies have not been conducted with bimekizumab-bkzx.
No effects on fertility parameters such as effects on reproductive organs, menstrual cycle length, or sperm analysis were observed in sexually mature cynomolgus monkeys that were subcutaneously administered 200 mg/kg/week bimekizumab-bkzx (150 times the MRHD, based on mg/kg comparison) for 26 weeks. The monkeys were not mated to evaluate fertility.
-
14 CLINICAL STUDIES14.1 Plaque Psoriasis - Three multicenter, randomized, double-blind trials [Trial Ps-1 (NCT03370133), Trial Ps-2 (NCT03410992), and Trial Ps-3 (NCT03412747)] enrolled a total of 1,480 subjects ...
14.1 Plaque Psoriasis
Three multicenter, randomized, double-blind trials [Trial Ps-1 (NCT03370133), Trial Ps-2 (NCT03410992), and Trial Ps-3 (NCT03412747)] enrolled a total of 1,480 subjects 18 years of age and older with moderate to severe plaque psoriasis who had a body surface area (BSA) involvement of ≥10%, an Investigator's Global Assessment (IGA) score of ≥3 ("moderate") in the overall assessment of psoriasis on a severity scale of 0 to 4, and a Psoriasis Area and Severity Index (PASI) score ≥12.
In Trial Ps-1, 567 subjects were randomized to receive either BIMZELX 320 mg by subcutaneous injection every 4 weeks, ustekinumab (for subjects weighing ≤100kg, 45 mg initially and 4 weeks later, then every 12 weeks; for subjects weighing >100kg, 90 mg initially and 4 weeks later, then every 12 weeks), or placebo through Week 52. At Week 16, subjects originally randomized to placebo received BIMZELX 320 mg every 4 weeks through Week 52.
In Trial Ps-2, 435 subjects were randomized to either BIMZELX 320 mg by subcutaneous injection every 4 weeks or placebo. At Week 16, subjects who achieved a PASI 90 response continued into a 40-week randomized withdrawal period. Subjects originally randomized to BIMZELX 320 mg every 4 weeks were re-randomized to either BIMZELX 320 mg every 4 weeks or BIMZELX 320 mg every 8 weeks or placebo. Subjects originally randomized to placebo continued to receive placebo if they were PASI 90 responders. Subjects who did not achieve a PASI 90 response at week 16 entered an open-label escape arm and received BIMZELX 320 mg every 4 weeks for 12 weeks. Subjects who relapsed, defined as having a less than PASI 75 response compared to baseline, during the randomized withdrawal period also entered the 12-week escape arm.
In Trial Ps-3, 478 subjects were randomized to receive either BIMZELX 320 mg by subcutaneous injection every 4 weeks through week 56, BIMZELX 320 mg every 4 weeks through week 16 followed by BIMZELX every 8 weeks through week 56, or adalimumab (80 mg as an initial dose followed by 40 mg every other week starting 1 week after initial dose through Week 24) followed by BIMZELX 320 mg every 4 weeks through Week 56.
In Trial Ps-1, Trial Ps-2, and Trial Ps-3, 71% of the subjects were male and 84% of the subjects were White, with a mean age of 45 years and a mean weight of 89 kg. At baseline, subjects had a median baseline PASI score of 18, median baseline for BSA of 20%, and baseline IGA score of 4 ("severe") in 33% of subjects. A total of 93% subjects had psoriasis of the scalp (Scalp IGA score of ≥1) and a total of 26% of subjects had a history of psoriatic arthritis. Additionally, 38% had received prior biologic therapy.
Clinical Response at Week 16 (Trial Ps-1 and Trial Ps-2)
Trial Ps-1 and Trial Ps-2 responses at Week 16 compared to placebo for the two co-primary endpoints:
- The proportion of subjects who achieved an IGA score of 0 ("clear") or 1 ("almost clear") with at least a 2-grade improvement from baseline
- The proportion of subjects who achieved at least a 90% reduction from baseline PASI (PASI 90)
Secondary endpoints included the proportion of subjects who achieved PASI 100, IGA 0, and Scalp IGA response (defined as Scalp IGA score of 0 [clear] or 1 [almost clear] with at least 2-grade of improvement from baseline) at Week 16, and PASI 75 at Week 4. In addition, secondary endpoints included assessment of psoriasis symptoms (itching, pain, and scaling) measured by the Patient Symptom Diary (PSD) at Week 16.
The proportion of subjects who achieved IGA 0 or 1, PASI 90, IGA 0, and PASI 100 response at Week 16 are presented in Table 3.
Table 3: Efficacy Results at Week 16 in BIMZELX or Placebo-Treated Adults with Plaque Psoriasis in Trial Ps-1 and Trial Ps-2 Trial Ps-1 Trial Ps-2 BIMZELX 320 mg every 4 weeks
(N=321)
n (%)Placebo
(N=83)
n (%)BIMZELX 320 mg every 4 weeks
(N=349)
n (%)Placebo
(N=86)
n (%)- *
- Co-primary endpoints
IGA 0 or 1 ("clear" or "almost clear")* 270 (84%) 4 (5%) 323 (93%) 1 (1%) Difference (95% CI) 79% (73%, 85%) 91% (88%, 95%) PASI 90* 273 (85%) 4 (5%) 317 (91%) 1 (1%) Difference (95% CI) 80% (74%, 86%) 90% (86%, 93%) IGA 0 ("clear") 188 (59%) 0 (0%) 243 (70%) 1 (1%) Difference (95% CI) 59% (53%, 64%) 69% (64%, 74%) PASI 100 188 (59%) 0 (0%) 238 (68%) 1 (1%) Difference (95% CI) 59% (53%, 64%) 67% (62%, 73%) Examination of age, gender, race, baseline IGA score and previous treatment with systemic or biologic agents did not identify differences in response to BIMZELX among these subgroups at Week 16.
A greater proportion of subjects randomized to BIMZELX achieved PASI 75 at Week 4 in both trials compared to placebo. In Trial Ps-1, 77% of subjects treated with BIMZELX achieved PASI 75 compared to 2% treated with placebo. In Trial Ps-2, 76% of subjects treated with BIMZELX achieved PASI 75 compared to 1% treated with placebo.
Among subjects with Scalp IGA score of at least 2 at baseline, a greater proportion of subjects randomized to BIMZELX achieved Scalp IGA response at Week 16 in both trials compared to placebo. In Trial Ps-1, 84% (240/285) of subjects treated with BIMZELX achieved Scalp IGA response compared to 15% (11/72) of placebo treated subjects. In Trial Ps-2, 92% (286/310) of subjects treated with BIMZELX achieved Scalp IGA response compared to 7% (5/74) of placebo treated subjects.
Maintenance and Durability of Response
In Trial Ps-2, subjects randomized to BIMZELX every 4 weeks at Week 0 and who were PASI 90 responders at Week 16 were re-randomized to either continue treatment with BIMZELX every 4 weeks, switched to BIMZELX every 8 weeks, or be withdrawn from therapy (i.e., received placebo).
Figure 1 and Figure 2 present the percentage of subjects maintaining IGA score of 0 ("Clear") or 1 ("Almost Clear") and PASI 90, respectively, through Week 56 after re-randomization at Week 16.
Figure 1: Percentage of Subjects Maintaining IGA 0 or 1 through Week 56 after Re-Randomization at Week 16
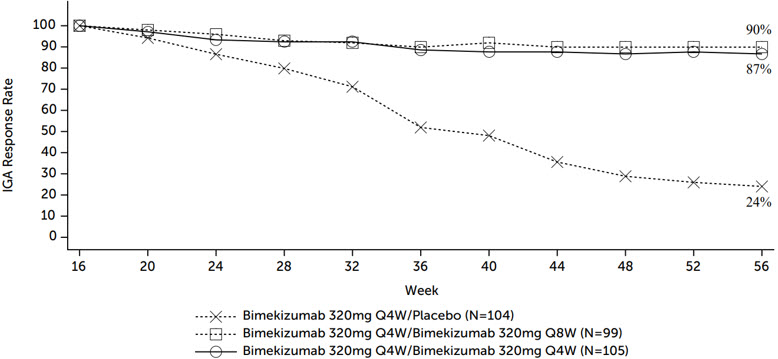
For IGA 0 or 1 responders at Week 16 who were re-randomized to treatment withdrawal (i.e., placebo), the median time to loss of IGA 0 or 1 response was approximately 24 weeks. Among these subjects with IGA score of 2 at retreatment, 58% (14/24) achieved IGA score of 0 within 12 weeks of restarting treatment with BIMZELX 320 mg every 4 weeks. Among these subjects with IGA score ≥ 3 at retreatment, 87% (34/39) regained IGA 0 or 1 response with at least 2-grade improvement from retreatment within 12 weeks of restarting treatment with BIMZELX 320 mg every 4 weeks.
Figure 2: Percentage of Subjects Maintaining PASI 90 through Week 56 after Re-Randomization at Week 16
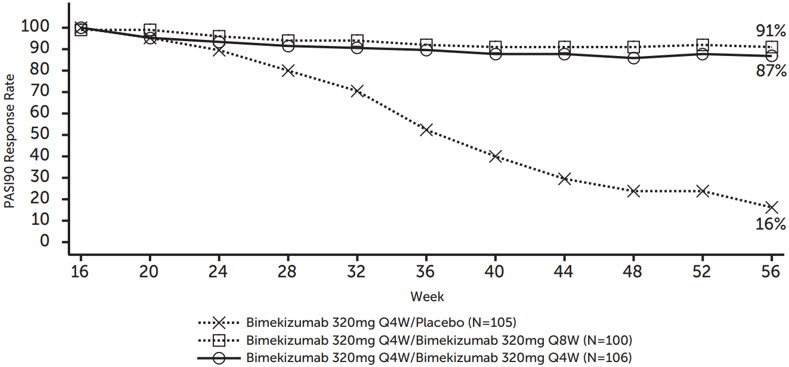
For PASI 90 responders at Week 16 who were re-randomized to treatment withdrawal (i.e., placebo), the median time to loss of PASI 90 response was approximately 24 weeks.
14.2 Psoriatic Arthritis
The safety and efficacy of BIMZELX were assessed in 1,112 subjects in two multicenter, randomized, double-blind, placebo-controlled studies [Trial PsA-1 (NCT 03895203) and Trial PsA-2 (NCT 03896581)] in subjects 18 years and older with active psoriatic arthritis (PsA).
Subjects in these studies had a diagnosis of PsA of at least 6 months based on Classification Criteria for Psoriatic Arthritis (CASPAR), a median duration of 4.6 years at baseline, and active disease with ≥3 tender joint count and ≥3 swollen joint count. Subjects with each subtype of PsA were enrolled in these studies, including polyarticular symmetric arthritis (63.5%), oligoarticular asymmetric arthritis (25.9%), distal interphalangeal joint predominant (4.4%), spondylitis predominant (4.2%), and arthritis mutilans (1.5%). At baseline, 56% of subjects had ≥3% Body Surface Area (BSA) with active plaque psoriasis. At baseline across both studies, 32% and 12% of subjects had enthesitis and dactylitis, respectively, 58% of subjects had psoriatic nail disease, and 53% of subjects were receiving concomitant methotrexate.
The PsA-1 study evaluated 852 biologic-naïve subjects, who were treated with either BIMZELX 160 mg every 4 weeks up to Week 52, adalimumab 40 mg every 2 weeks up to Week 52 (active reference arm), or placebo. Subjects receiving placebo were switched to BIMZELX every 4 weeks at Week 16 to Week 52. In this study, 78% of subjects had received prior treatment with ≥ 1 conventional DMARDs (cDMARDs), and 22 % of subjects had no prior treatment with cDMARDs. At baseline, 58% of subjects were receiving concomitant methotrexate (MTX), 11% were receiving concomitant cDMARDs other than MTX, and 31% were receiving no cDMARDs. The PsA-2 study evaluated 400 anti-TNFα experienced subjects (inadequate response or intolerance to treatment), who were treated with BIMZELX 160 mg every 4 weeks or placebo up to Week 16. In this study, 43% of subjects were receiving concomitant MTX, 8% were receiving concomitant cDMARDs other than MTX, and 50% were receiving no cDMARDs.
For both studies, the primary endpoint was the proportion of subjects who achieved an America College of Rheumatology (ACR) 50 response at Week 16.
Clinical Response
In both studies, treatment with BIMZELX resulted in significant improvement in disease activity, as measured by ACR, compared to placebo at Week 16 (see Table 4). Responses in Trial PsA-2 (anti-TNF experienced) were similar to Trial PsA-1.
Table 4: Clinical Responses at Week 16 in Trial PsA-1 and Trial PsA-2 Trial PsA-1 – bDMARD naïve Trial PsA-2 – anti-TNFα experienced Endpoint BIMZELX 160 mg Q4W
N=431
n (%)Placebo
N=281
n (%)Difference from Placebo*
(95% CI)BIMZELX 160 mg Q4W
N=267
n (%)Placebo
N=133
n (%)Difference from Placebo*
(95% CI)CI= confidence interval ACR 20 Response Week 16 268 (62.2) 67 (23.8) 38.3 (31.6, 45.1) 179 (67.0) 21 (15.8) 51.3 (42.9, 59.6) ACR 50 Response Week 16 189 (43.9)† 28 (10.0) 33.9 (28.0, 39.7) 116 (43.4)† 9 (6.8) 36.7 (29.4, 44.0) ACR 70 Response Week 16 105 (24.4) 12 (4.3) 20.1 (15.4, 24.8) 71 (26.6) 1 (0.8) 25.8 (15.6, 35.7)‡ The percentage of subjects achieving ACR50 responses in Trial PsA-1 by visit through Week 16 is shown in Figure 3. Similar responses were seen in Trial PsA-2 up to Week 16.
Figure 3: Percent of Subjects Achieving ACR 50 Responses in Trial PsA-1 through Week 16
The results of the components of the ACR response criteria are shown in Table 5.
Table 5: Mean change from Baseline in ACR Component Scores at Week 16 in Trial PsA-1 and Trial PsA-2 Trial PsA-1 – bDMARD naïve Trial PsA-2 – anti-TNFα experienced Placebo
(N=281)BIMZELX 160 mg Q4W
(N=431)Placebo
(N=133)BIMZELX 160 mg Q4W
(N=267)Multiple Imputation (MI) is used for all endpoints presented in Table 5. - *
- p<0.001 reference-based imputation versus placebo adjusted for multiplicity.
Number of Swollen Joints Baseline 9.5 9.0 10.3 9.7 Mean change at Week 16 -3.0 -6.6 -2.0 -7.0 Number of Tender Joints Baseline 17.1 16.8 19.3 18.4 Mean change at Week 16 -3.2 -10.0 -2.4 -10.9 Patient's Assessment of Pain Baseline 56.8 53.7 61.7 58.3 Mean change at Week 16 -6.2 -23.6 -4.5 -27.7 Patient's Global Assessment Baseline 58.6 54.4 63.0 60.5 Mean change at Week 16 -7.7 -26.3 -5.5 -31.8 Physician Global Assessment Baseline 57.3 57.2 57.7 59.3 Mean change at Week 16 -12.5 -37.4 -6.8 -49.4 Health Assessment Questionnaire- Disability Index (HAQ-DI) Baseline 0.9 0.8 1.0 1.0 Mean Change at Week 16 -0.1 -0.3* -0.1 -0.4* High Sensitivity C-reactive Protein (hsCRP) mg/L Baseline 11.4 8.7 11.6 12.4 Mean Change at Week 16 -2.4 -4.2 3.6 -7.0 Treatment with BIMZELX resulted in improvement in dactylitis and enthesitis in subjects with pre-existing dactylitis or enthesitis, compared to placebo.
In subjects with coexistent plaque psoriasis receiving BIMZELX, the skin lesions of psoriasis improved with treatment, relative to placebo, as measured by the Psoriasis Area Severity Index (PASI 90) at Week 16.
Radiographic Response
In Trial PsA-1, inhibition of progression of structural damage was assessed radiographically and expressed as the change from baseline in the Van der Heijde modified total Sharp Score (vdHmTSS) and its components, the Erosion Score (ES) and the Joint Space Narrowing score (JSN), at Week 16 (see Table 6).
BIMZELX significantly inhibited the rate of progression of joint damage at Week 16 in the overall population compared to placebo. The change from Baseline in erosion subscores contributed more to the change from Baseline in vdHmTSS total score than the change from Baseline in joint narrowing subscore. The percentage of subjects with no radiographic joint damage progression (defined as a change from baseline in mTSS of ≤0.0) from randomization to Week 16 was 77% for BIMZELX and 69% for placebo in the overall population. Similar responses were achieved in the population with elevated hsCRP and/or at least 1 bone erosion (75% for BIMZELX and 67% for placebo).
Physical Function
In both studies, subjects treated with BIMZELX showed statistically significant improvement from baseline in physical function compared with placebo as assessed by HAQ-DI at Week 16 (see Table 5). In both studies, a greater proportion of subjects achieved a reduction of at least 0.35 in HAQ-DI score from baseline in the BIMZELX group compared with placebo at Week 16.
14.3 Non-Radiographic Axial Spondyloarthritis
The efficacy and safety were assessed in 254 patients in one randomized, double-blind, placebo-controlled study [Trial nr-axSpA-1 (NCT03928704)] in adult subjects 18 years of age and older with active non-radiographic axial spondyloarthritis. Subjects had to have objective signs of inflammation with elevated C-reactive protein (CRP) level and/or evidence of sacroiliitis on Magnet Resonance Imaging (MRI). Subjects met ASAS classification criteria for axial spondyloarthritis and have active disease as defined by BASDAI greater than or equal to 4, spinal pain of greater than or equal to 4 (0-10 numeric rating scale (NRS)), and no definitive radiographic evidence of structural damage in the sacroiliac joints. At baseline, 73% of subjects had enthesitis. Subjects also had a history of inadequate response to 2 different non-steroidal anti-inflammatory drugs (NSAIDs), or intolerance or contraindication to NSAIDs. Approximately 24% of subjects were on concomitant cDMARDs. Overall, 11% of subjects had received previous treatment (failed or were intolerant to) with anti-TNF alpha agents.
Subjects were randomized to receive BIMZELX 160 mg or placebo every 4 weeks up to the completion of Week 16 assessments. Starting at Week 16, all subjects received BIMZELX every 4 weeks up to Week 52. The primary endpoint was at least 40% improvement in Assessment of Spondyloarthritis International Society (ASAS 40) at Week 16.
Clinical Response
In Trial nr-axSpA-1, treatment with BIMZELX resulted in significant improvements in the measure of disease activity compared to placebo at Week 16 (Table 7).
Table 7: Clinical Response in Trial nr-axSpA-1 at Week 16 BIMZELX 160 mg Q4W
(N=128)Placebo
(N=126)Difference from placebo (95% CI)* n (%) n (%) NRI is used
CI= confidence intervalASAS 40 response 61 (47.7%)† 27 (21.4%) 26.2 (15.0, 37.5) ASAS 20 response 88 (68.8%)† 48 (38.1%) 30.7 (19.0, 42.3) Similar responses were seen regardless of prior anti-TNF alpha therapy. Treatment with BIMZELX resulted in improvement in enthesitis in subjects with pre-existing enthesitis.
The results of the main components of the ASAS 40 response criteria and other measures of disease activity are shown in Table 8.
Table 8: Components of the ASAS 40 Response Criteria and Other Measures of Disease Activity in nr-axSpA Subjects at Baseline and Week 16 in Trial nr-axSpA-1 BIMZELX 160 mg Q4W
(N= 128)Placebo
(N=126)BASFI = Bath Ankylosing Spondylitis Functional Index BASMI = Bath Ankylosing Spondylitis Metrology Index BASDAI = Bath Ankylosing Spondylitis Disease Activity Index MI is used for all endpoints presented in Table 8 ASAS Components - -
- Patient Global Assessment (0-10)
Baseline 7.1 6.9 Mean Change from Baseline -3.2 -1.4 - -
- Total Spinal Pain (0-10)
Baseline 7.3 7.1 Mean Change from Baseline -3.4 -1.7 - -
- BASFI (0-10)
Baseline 5.5 -2.5* Mean Change from Baseline 5.3 -1.0 - -
- Inflammation (0-10)†
Baseline 7.0 6.9 Mean Change from Baseline -3.6 -1.9 Other Measures of Disease Activity BASDAI Score
Baseline 6.9 6.7 Mean Change from Baseline -3.1* -1.5 BASMI
Baseline 2.9 3.0 Mean Change from Baseline -0.4 -0.1 hsCRP (mg/L)
Baseline 11.1 10.2 Mean Change from Baseline -6.7 0.0 14.4 Ankylosing Spondylitis
The efficacy and safety were assessed in 332 patients in one randomized, double-blind, placebo-controlled study [Trial AS-1 (NCT03928743)] in adult subjects 18 years of age and older with active ankylosing spondylitis. Subjects had to have documented radiologic evidence (x-ray) fulfilling the Modified New York criteria for AS. Subjects had active disease as defined by BASDAI ≥4 and spinal pain ≥4 on a 0 to 10 numeric rating scale (NRS) (from BASDAI Item 2). Subjects also had a history of inadequate response to 2 different non-steroidal anti-inflammatory drugs (NSAIDs), or intolerance or contraindication to NSAIDs. Approximately 20% of subjects were on concomitant cDMARDs. Overall, 16% of subjects had received previous treatment (failed or were intolerant to) with anti-TNF alpha agents.
Subjects were randomized 2:1 to receive BIMZELX 160 mg or placebo every 4 weeks up to the completion of Week 16 assessments. Starting at Week 16, all subjects received BIMZELX every 4 weeks up to Week 52. The primary endpoint was at least 40% improvement in Assessment of Spondyloarthritis International Society (ASAS 40) at Week 16.
Clinical Response
In Trial AS-1, treatment with BIMZELX resulted in significant improvements in the measure of disease activity compared to placebo at Week 16 (Table 9).
Table 9: Clinical Response in Trial AS-1 at Week 16 BIMZELX 160 mg Q4W
(N=221)Placebo
(N=111)Difference from placebo (95% CI)* n (%) n (%) NRI is used
CI= confidence intervalASAS 40 response 99 (44.8%)† 25 (22.5%) 22.3 (12.1, 32.4) ASAS 20 response 146 (66.1%)† 48 (43.2%) 22.8 (11.7, 34.0) Similar responses were seen regardless of prior anti-TNF alpha therapy. Treatment with BIMZELX resulted in improvement in enthesitis in subjects with pre-existing enthesitis.
The results of the main components of the ASAS 40 response criteria and other measures of disease activity are shown in Table 10.
Table 10: Components of the ASAS 40 Response Criteria and Other Measures of Disease Activity in Ankylosing Spondylitis Subjects at Baseline and Week 16 in Trial AS-1 BIMZELX 160 mg Q4W
(N= 221)Placebo
(N=111)BASFI = Bath Ankylosing Spondylitis Functional Index BASMI = Bath Ankylosing Spondylitis Metrology Index BASDAI = Bath Ankylosing Spondylitis Disease Activity Index MI is used for all endpoints presented in Table 10 ASAS Components - -
- Patient Global Assessment (0-10)
Baseline 6.6 6.7 Mean Change from Baseline -2.7 -1.6 - -
- Total Spinal Pain (0-10)
Baseline 7.1 7.2 Mean Change from Baseline -3.3 -1.9 - -
- BASFI (0-10)
Baseline 5.3 5.2 Mean Change from Baseline -2.2* -1.1 - -
- Inflammation (0-10)†
Baseline 6.7 6.8 Mean Change from Baseline -3.2 -2.1 Other Measures of Disease Activity BASDAI Score
Baseline 6.4 6.5 Mean Change from Baseline -2.9* -1.9 BASMI
Baseline 3.9 3.8 Mean Change from Baseline -0.5‡ -0.2 hsCRP (mg/L)
Baseline 14.7 13.6 Mean Change from Baseline -8.6 -2.2 The percentage of subjects achieving ASAS 40 responses in Trial AS-1 by visit through Week 16 is shown in Figure 5.
Figure 5: Percent of Subjects Achieving ASAS 40 Responses in Trial AS-1 Through Week 16
 Close
Close14.5 Hidradenitis Suppurativa
The safety and efficacy of BIMZELX were assessed in two Phase 3 multicenter, randomized, double-blind, placebo-controlled trials [Trial HS-1 (NCT04242446) and Trial HS-2 (NCT04242498)] in 1,014 adult subjects with moderate to severe HS of at least 6 months with Hurley Stage II or Hurley Stage III disease, and with ≥5 inflammatory lesions [i.e., number of abscesses plus number of inflammatory nodules (AN count)], and a history of inadequate response to a course of systemic antibiotics for the treatment of HS.
Subjects received BIMZELX 320 mg every 2 weeks (Q2W) for 48 weeks, or BIMZELX 320 mg every 4 weeks (Q4W) up to Week 48, or BIMZELX 320 mg Q2W to Week 16, followed by 320 mg Q4W up to Week 48, or placebo. At Week 16, subjects receiving placebo were switched to BIMZELX 320 mg Q2W to Week 48. Concomitant oral doxycycline, minocycline, or an equivalent systemic tetracycline for HS was allowed if the subject was on a stable dose regimen for 28 days prior to baseline.
In these trials, at baseline the mean age of all subjects was 37 years, 57% of subjects were female, 80% were White, 11% were Black or African American, and 4% were Asian; for ethnicity, 7% identified as Hispanic or Latino. Of the subjects enrolled in trials conducted in the United States, 33% were Black or African American. The mean BMI was 33, and 46% were current smokers. Subjects had a median disease duration of 5 years. Overall, 9% of subjects were receiving concomitant antibiotic therapy for HS, and 19% of subjects had received previous treatment with biologics. The proportion of Hurley Stage II and Stage III subjects were 56% and 44%, respectively. Additionally, the mean number of draining tunnels was 3.6 and mean AN count was 16.3.
The primary efficacy endpoint in both trials was the Hidradenitis Suppurativa Clinical Response 50 (HiSCR50) at Week 16, defined by at least a 50% reduction in total abscess and inflammatory nodule count with no increase in abscess or draining tunnel count relative to baseline. Secondary endpoints included the proportion of subjects who achieved HiSCR75 and HS-specific skin pain response as assessed by a 0 to 10 numeric rating scale (NRS).
Clinical Response at Week 16 (Trials HS-1 and HS-2)
In both trials, a higher proportion of BIMZELX-treated subjects achieved HiSCR50 and HiSCR75 compared to placebo (see Table 11).
Table 11: Efficacy Results in Adults with HS in Trials HS-1 and HS-2 at Week 16 * Trial HS-1 Trial HS-2 BIMZELX
320mg Q2W
(N=289)Placebo
(N=72)BIMZELX
320 mg Q2W
(N=291)Placebo
(N=74)- *
- Subjects who initiated systemic antibiotics (new antibiotic or change in the dose/type of current antibiotic) for any reason or who discontinued due to adverse event or lack of efficacy are treated as non-responders at all subsequent visits. Other missing data were imputed via multiple imputation.
HiSCR50 48% 29% 52% 32% Difference (95% CI) 18% (6%, 30%) 20% (8%, 32%) HiSCR75 33% 18% 36% 16% Difference (95% CI) 15% (4%, 27%) 20% (10%, 31%) Examination of age, gender, race, disease duration, weight, prior biologic therapy, systemic antibiotic use at baseline, Hurley stage, and smoking status did not identify meaningful differences in response to BIMZELX among these subgroups at Week 16.
Figure 6 presents the proportion of subjects achieving HiSCR50 response in Trials HS-1 and HS-2 by visit through Week 16.
Figure 6: HiSCR50 Response through Week 16 in Adults with HS in Trials HS-1 and HS-2

Subjects who initiated systemic antibiotics (new antibiotic or change in the dose/type of current antibiotic) for any reason through Week 16 or who discontinued due to adverse event or lack of efficacy are treated as non-responders at all subsequent visits. Other missing data were imputed via multiple imputation.
BIMZELX was associated with an improvement in patient-reported worst skin pain (lesion pain) based on the achievement of a reduction of at least 3 points (as measured on a 0 to 10 NRS) compared to placebo at Week 16.
-
16 HOW SUPPLIED/STORAGE AND HANDLINGHow Supplied - BIMZELX (bimekizumab-bkzx) injection is a sterile, preservative-free, clear to slightly opalescent, and colorless to pale brownish-yellow solution. Each prefilled autoinjector or ...
How Supplied
BIMZELX (bimekizumab-bkzx) injection is a sterile, preservative-free, clear to slightly opalescent, and colorless to pale brownish-yellow solution. Each prefilled autoinjector or prefilled syringe contains 1 mL or 2 mL of a 160 mg/mL solution. The autoinjectors and prefilled syringes are not made with natural rubber latex. BIMZELX is supplied as:
BIMZELX autoinjector:
- NDC 50474-781-85: Carton of two 160 mg/mL single-dose autoinjectors. Each prefilled autoinjector is fixed with a 27 gauge ½ inch needle.
- NDC 50474-781-84: Carton of one 160 mg/mL single-dose autoinjector. The prefilled autoinjector is fixed with a 27 gauge ½ inch needle.
- NDC 50474-782-84: Carton of one 320 mg/2 mL (160 mg/mL) single-dose autoinjector fixed with a 27 gauge ½ inch needle.
BIMZELX prefilled syringe:
- NDC 50474-780-79: Carton of two 160 mg/mL single-dose prefilled syringes. Each prefilled syringe is fixed with a 27 gauge ½ inch needle with needle guard.
- NDC 50474-780-78: Carton of one 160 mg/mL single dose prefilled syringe. The prefilled syringe is fixed with a 27 gauge ½ inch needle with a needle guard.
- NDC 50474-783-78: Carton of one 320 mg/2 mL (160 mg/mL) single-dose prefilled syringe with a 27 gauge ½ inch needle.
CloseStorage and Handling
Store cartons with BIMZELX refrigerated between 2°C to 8°C (36°F to 46°F). Keep the product in the original carton to protect it from light until the time of use. Do not freeze. Do not shake. Do not use beyond expiration date. BIMZELX does not contain a preservative; discard any unused portion.
When necessary, BIMZELX prefilled syringes or autoinjectors may be stored at room temperature up to 25°C (77°F) in the original carton for a single period of up to 30 days. Once BIMZELX prefilled syringes or autoinjectors have been stored at room temperature, do not place back in refrigerator. Write the date removed from the refrigerator in the space provided on the carton and discard if not used within a 30-day period.
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient and/or caregiver to read the FDA-approved patient labeling (Medication Guide and Instructions for Use). Administration Instructions - Instruct patients or caregivers to ...
Advise the patient and/or caregiver to read the FDA-approved patient labeling (Medication Guide and Instructions for Use).
Administration Instructions
Instruct patients or caregivers to perform the first self-injection under the supervision and guidance of a qualified healthcare professional for proper training in subcutaneous injection technique [see Dosage and Administration (2.9), Instructions for Use].
If two separate 160 mg injections are used to achieve the recommended dose, instruct patients or caregivers to administer each injection subcutaneously at a different anatomic location [see Dosage and Administration (2.9)].
Instruct patients or caregivers in the technique of needle and syringe disposal [see Instructions for Use].
Advise patients if they forget to take their dose of BIMZELX to inject their dose as soon as they remember. Instruct patients to then take their next dose at the appropriate scheduled time [see Dosage and Administration (2.7)].
Suicidal Ideation and Behavior
Instruct patients and their caregivers to monitor for the emergence of suicidal ideation and behavior and promptly seek medical attention if the patient experiences suicidal ideation or behavior; or new onset or worsening depression, anxiety, or other mood changes. Instruct patients to call the National Suicide and Crisis Lifeline at 988 if they experience suicidal ideation or behavior [see Warnings and Precautions (5.1)].
Infections
Inform patients that BIMZELX may lower the ability of their immune system to fight infections. Instruct patients of the importance of communicating any history of infections to the healthcare provider and contacting their healthcare provider if they develop any symptoms of an infection [see Warnings and Precautions (5.2)].
Liver Biochemical Abnormalities
Inform patients that BIMZELX may increase the risk of elevated liver enzymes. Acute liver disease or cirrhosis may increase this risk. Advise patients that laboratory evaluation is needed prior to and periodically during treatment. Advise patients to hold the next dose of BIMZELX and call their healthcare provider right away, if signs or symptoms of liver dysfunction occur [see Warnings and Precautions (5.4)].
Inflammatory Bowel Disease
Instruct patients to seek medical advice if they develop signs and symptoms of Crohn's disease or ulcerative colitis [see Warnings and Precautions (5.5)].
Immunizations
Advise patients to avoid vaccination with live vaccines during BIMZELX treatment. Instruct patients to inform their healthcare practitioner that they are taking BIMZELX prior to a potential vaccination [see Warnings and Precautions (5.6)].
ClosePregnancy
Advise patients that there is a pregnancy registry that monitors pregnancy outcomes in patients exposed to BIMZELX during pregnancy [see Use in Specific Populations (8.1)].
-
SPL UNCLASSIFIED SECTIONManufactured by: UCB, Inc. 1950 Lake Park Drive Smyrna, GA 30080 - US License No. 1736
-
MEDICATION GUIDEMEDICATION GUIDE - BIMZELX® (bim zel'ex) (bimekizumab-bkzx) injection, for subcutaneous use - This Medication Guide has been approved by the U.S. Food and Drug Administration.Revised ...Close
MEDICATION GUIDE
BIMZELX® (bim zel'ex)
(bimekizumab-bkzx)
injection, for subcutaneous useThis Medication Guide has been approved by the U.S. Food and Drug Administration. Revised: November 2024 What is the most important information I should know about BIMZELX?
BIMZELX is a medicine that affects your immune system. BIMZELX may increase your risk of having serious side effects, including:-
Suicidal thoughts and behavior. New or worsening suicidal thoughts and behavior have happened in some people treated with BIMZELX. Get medical help right away or call the National Suicide and Crisis Lifeline at 988 if you, your caregiver or your family member notice in you any of the following symptoms:
- new or worsening depression or anxiety
- thoughts of suicide, dying, or hurting yourself
- changes in behavior or mood
- acting on dangerous impulses
- attempt to commit suicide
-
Infections. BIMZELX is a medicine that may lower the ability of your immune system to fight infections and may increase your risk of infections, including serious infections.
- Your healthcare provider should check you for infections and tuberculosis (TB) before starting treatment with BIMZELX.
- If your healthcare provider feels you are at risk for TB, you may be treated with medicine for TB before you begin treatment with BIMZELX and during your treatment with BIMZELX.
- Your healthcare provider should watch you closely for signs and symptoms of TB during and after treatment with BIMZELX. Do not take BIMZELX if you have an active TB infection.
- are being treated for an infection
- have an infection that does not go away or keeps coming back
- have TB or have been in close contact with someone with TB
- think you have an infection or have symptoms of an infection such as:
- fever, sweats, or chills
- muscle aches
- cough
- shortness of breath
- blood in your phlegm
- weight loss
- warm, red, or painful skin or sores on your body different from your psoriasis
- diarrhea or stomach pain
- burning when you urinate or urinating more often than normal
After starting BIMZELX, call your healthcare provider right away if you have any of the signs of infection listed above. Do not use BIMZELX if you have any signs of infection unless you are instructed to by your healthcare provider.
See "What are the possible side effects of BIMZELX?" for more information about side effects.What is BIMZELX?
BIMZELX is a prescription medicine used to treat:- adults with moderate to severe plaque psoriasis who may benefit from taking injections or pills (systemic therapy) or treatment using ultraviolet light alone or with pills (phototherapy).
- adults with active psoriatic arthritis.
- adults with active non-radiographic axial spondyloarthritis with objective signs of inflammation.
- adults with active ankylosing spondylitis.
- adults with moderate to severe hidradenitis suppurativa.
Before using BIMZELX, tell your healthcare provider about all of your medical conditions, including if you: - have any of the conditions or symptoms listed in the section "What is the most important information I should know about BIMZELX?"
- have a history of depression, or suicidal thoughts or behavior
- have liver problems
- have inflammatory bowel disease (Crohn's disease or ulcerative colitis)
- have recently received or are scheduled to receive an immunization (vaccine). You should avoid receiving live vaccines during treatment with BIMZELX. Tell all your healthcare providers that you are being treated with BIMZELX before receiving a vaccine.
- are pregnant or plan to become pregnant. It is not known if BIMZELX can harm your unborn baby.
- If you become pregnant while taking BIMZELX, you are encouraged to enroll in the Pregnancy Registry. The purpose of the pregnancy registry is to collect information about the health of you and your baby. Talk to your healthcare provider or call 1-877-311-8972 to enroll in this registry or visit http://mothertobaby.org/pregnancy-studies/.
- are breastfeeding or plan to breastfeed. It is not known if BIMZELX passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby during treatment with BIMZELX.
How should I use BIMZELX?
See the detailed "Instructions for Use" that comes with your BIMZELX for information on how to prepare and inject a dose of BIMZELX, and how to properly throw away (dispose of) used BIMZELX autoinjectors and prefilled syringes.- Use BIMZELX exactly as prescribed by your healthcare provider.
- If you miss your BIMZELX dose, inject a dose as soon as you remember. Then, take your next dose at your regular scheduled time. Call your healthcare provider if you are not sure what to do.
What are the possible side effects of BIMZELX?
BIMZELX may cause serious side effects, including:- See "What is important information I should know about BIMZELX?"
- Elevated liver enzyme levels. Your healthcare provider will do blood tests to check your liver enzyme levels before starting treatment and during treatment with BIMZELX. Your healthcare provider may temporarily stop or permanently stop your treatment with BIMZELX if you develop liver problems. Call your healthcare provider right away if you develop any signs or symptoms of liver problems, including:
- pain on the right side of your stomach-area
- feeling very tired
- loss of appetite
- nausea and vomiting
- itching
- dark urine
- light-colored stool
- yellowing of your skin or the whites of your eyes
- Inflammatory bowel disease. New cases of inflammatory bowel disease or "flare-ups" have happened with BIMZELX. If you have inflammatory bowel disease (Crohn's disease or ulcerative colitis), tell your healthcare provider if you have worsening disease symptoms during treatment with BIMZELX or develop new symptoms of stomach pain or diarrhea. Your healthcare provider will stop treatment with BIMZELX if you develop new or worsening signs of Crohn's disease or ulcerative colitis.
The most common side effects of BIMZELX in people treated for Psoriasis and Hidradenitis Suppurativa include: - upper respiratory tract infections
- headache
- herpes simplex infections (cold sores in or around the mouth)
- small red bumps on your skin
- feeling tired
- fungal infections (oral thrush or infections in the mouth, throat, skin, nails, feet or genitals)
- pain, redness or swelling at injection site
- stomach flu (gastroenteritis)
- acne
The most common side effects of BIMZELX in people treated for psoriatic arthritis include: - upper respiratory tract infections
- headache
- urinary tract infection
- oral thrush or infections in the mouth
- diarrhea
The most common side effects of BIMZELX in people treated for non-radiographic axial spondyloarthritis include: - upper respiratory tract infections
- headache
- cough
- joint pain
- tonsilitis
- urinary tract infection
- oral thrush or infections in the mouth
- diarrhea
- feeling tired
- muscle aches
- liver enzyme increase
The most common side effects of BIMZELX in people treated for ankylosing spondylitis include: - upper respiratory tract infections
- headache
- pain at injection site
- vaginal yeast infections
- oral thrush or infections in the mouth
- diarrhea
- rash
These are not all of the possible side effects of BIMZELX.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.How should I store BIMZELX? - Store BIMZELX in the refrigerator between 36°F to 46°F (2°C to 8°C).
- BIMZELX may be stored at room temperature up to 77°F (25°C) for up to 30 days in the original carton. Do not place BIMZELX prefilled syringes or autoinjectors back in the refrigerator after they have been stored at room temperature.
- Write the date removed from the refrigerator in the space provided on the carton and throw away BIMZELX if it has been kept at room temperature and not been used within 30 days.
- Keep BIMZELX in the original carton until ready for use to protect from light.
- Do not freeze BIMZELX.
- Do not shake BIMZELX.
- Do not use BIMZELX past the expiration date printed on the carton.
Keep BIMZELX and all medicines out of the reach of children. General information about the safe and effective use of BIMZELX.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use BIMZELX for a condition for which it was not prescribed. Do not give BIMZELX to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about BIMZELX that is written for health professionals.What are the ingredients in BIMZELX?
Active ingredient: bimekizumab-bkzx
Inactive ingredients: glacial acetic acid, glycine, polysorbate 80, sodium acetate and Water for Injection, USP.
Manufactured by:
UCB, Inc.
1950 Lake Park Drive Smyrna, GA 30080
US License No. 1736
For more information, go to www.BIMZELX.com or call 1-844-599-2273 -
Suicidal thoughts and behavior. New or worsening suicidal thoughts and behavior have happened in some people treated with BIMZELX. Get medical help right away or call the National Suicide and Crisis Lifeline at 988 if you, your caregiver or your family member notice in you any of the following symptoms:
-
INSTRUCTIONS for USE BIMZELX® (bim zel' ex) (bimekizumab-bkzx) injection, for subcutaneous use single-dose autoinjectorThis Instructions for Use contains information on how to inject BIMZELX. Read this Instructions for Use before using the BIMZELX autoinjector and each time you get a refill. There may be new ...
This Instructions for Use contains information on how to inject BIMZELX.
Read this Instructions for Use before using the BIMZELX autoinjector and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or treatment.
These instructions are for 1 injection only. You may need more than 1 injection at a time depending on your prescribed dose of BIMZELX. Each BIMZELX autoinjector is for one-time (single-dose) use only.
Important Information you Need to Know Before Injecting BIMZELX:
- Your healthcare provider should show you or a caregiver how to prepare and inject BIMZELX using the autoinjector for the first time. Do not inject yourself or someone else until you have been shown how to inject BIMZELX the right way. Call your healthcare provider if you have any questions.
- These instructions are for 1 injection only.
- The BIMZELX autoinjector has a needle safety feature that will be activated to cover the needle after the injection is finished. The needle safety feature will help to prevent needle stick injuries to anyone who handles the autoinjector after injection.
- Do not share or reuse your BIMZELX autoinjector. You may give or get an infection.
- Do not remove the needle cap until just before you give the injection.
- If you have vision or hearing problems, do not use the BIMZELX autoinjector without help from a caregiver.
How should I store BIMZELX autoinjector?
- Store the BIMZELX autoinjector in the refrigerator between 36°F to 46°F (2°C to 8°C).
- BIMZELX autoinjectors may be stored at room temperature up to 77°F (25°C) in the original carton for up to 30 days. Do not place BIMZELX autoinjectors back in the refrigerator after they have been stored at room temperature.
- Write the date removed from the refrigerator in the space provided on the carton and throw away if it has been kept at room temperature and not used within 30 days.
- Keep BIMZELX in the original carton until ready for use to protect from light.
- Do not freeze BIMZELX.
- Do not shake BIMZELX.
- Do not use the BIMZELX autoinjector past the expiration date printed on the carton.
Keep BIMZELX autoinjectors and all medicines out of the reach of children.
BIMZELX autoinjector parts (see Figure A):
Supplies Needed for each BIMZELX injection
- 1 BIMZELX autoinjector. You may need 2 BIMZELX autoinjectors to give the prescribed dose.
Not provided in the BIMZELX autoinjector carton:
- 1 alcohol swab
- 1 clean cotton ball
- 1 sharps disposal container. See, "Step 12: Dispose of (throw away) the used BIMZELX autoinjector" at the end of this Instructions for Use.
Setting up for your BIMZELX injection.
Step 1: Take the BIMZELX autoinjector carton out of the refrigerator. Do not use the BIMZELX autoinjector(s) if the carton seal is broken. Throw it away and get a new one.
-
Keep the BIMZELX autoinjector in its original carton for 30 to 45 minutes to warm to room temperature. This will help to reduce discomfort when injecting.
- Do not microwave the autoinjector, run hot water over it, or leave it in direct sunlight.
- Do not shake the autoinjector.
- Do not take the cap off the autoinjector until you are ready to inject.
Step 2: Find a clean, flat, and well-lit work surface, like a table.
Step 3: Gather the supplies needed for your injection.
Step 4: Wash your hands well with soap and water and dry with a clean towel.
Step 5: Inspect the BIMZELX autoinjector (see Figure B):
- Remove the BIMZELX autoinjector from the carton.
-
Make sure the name BIMZELX appears on the label.
- Check the expiration date printed on the label.
- Check the medicine through the Viewing Window. The medicine inside should be clear to slightly pearly, and colorless to pale brownish-yellow. You may see air bubble(s) in the liquid. This is normal.
-
Do not use the BIMZELX autoinjector, throw it away and get a new one if:
- The expiration date printed on the label has passed.
- The medicine is cloudy, discolored, or has particles.
- It looks damaged or has been dropped.
Choose and prepare your injection site.
Step 6: Choose and clean your injection site.
- The sites you may choose for your injection are:
- Do not inject into areas where the skin is tender, bruised, red, hard, thick, scaly, or affected by psoriasis , or within 2 inches of the belly button (navel).
- Choose a new injection site on the body each time you use BIMZELX. Do not use the same injection site 2 times in a row.
Step 7: Prepare your skin
- Clean the injection site with an alcohol swab. Let the area dry completely. Do not touch the cleaned area again before injecting.
Injecting BIMZELX.
Step 8: Remove the BIMZELX autoinjector cap.
- Hold the BIMZELX autoinjector firmly with one hand around the handle. Pull the cap straight off (away from) the autoinjector with the other hand (see Figure E). Although you cannot see the needle tip, it is now uncovered.
- Put the cap into an FDA-cleared sharps disposal container right away. You will not need it again.
- Do not touch the needle guard or put the cap back on as it could activate the autoinjector and you can stick yourself.
Step 9: Hold the BIMZELX autoinjector at a 90-degree angle to the cleaned injection site (see Figure F).
Step 10: Place the BIMZELX autoinjector flat against your skin, then firmly press the BIMZELX autoinjector down against your skin. You will hear a "click" sound. Your injection begins when the 1st "click" is heard (see Figure G).
- Do not lift the autoinjector away from the skin.
Keep holding the BIMZELX autoinjector in place and pressed firmly against your skin. It will take about 15 seconds to receive your full dose.
- You will hear a 2nd "click" in about 15 seconds after you hear the first click.
- The second click tells you that all the medicine has been injected and your BIMZELX injection is finished. You should see the yellow color indicator filling the viewing window (see Figure H).
- Important: When you remove the autoinjector, if the viewing window has not turned yellow this means you may not have received a full dose. Call your healthcare provider right away.
Step 11: Remove the BIMZELX autoinjector by carefully pulling the BIMZELX autoinjector straight up from your skin. The needle guard will automatically cover the needle. Do not try to touch the needle.
- Press a clean cotton ball over the injection site for a few seconds. Do not rub the injection site. You may see slight bleeding or a drop of liquid. This is normal. You may cover the injection site with a small adhesive bandage, if needed.
Step 12: Dispose of (throw away) the used BIMZELX autoinjector (see Figure I).
- Put the used BIMZELX autoinjector in an FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) the BIMZELX autoinjector in your household trash. If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labeled to warn of hazardous waste inside the container.
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal.
-
Do not recycle your used sharps disposal container.
Step 13: Repeat steps 1 through 12 with a new BIMZELX autoinjector if you need to use 2 autoinjectors to give the prescribed dose.
- Make sure you select a new injection site on the body each time you use BIMZELX. Do not use the same site that you used for your first injection.
You can sign up to receive sharps containers for BIMZELX autoinjector disposal at no additional cost by going to www.BIMZELX.com or call 1-844-599-2273.
Manufactured by:
UCB, Inc. 1950 Lake Park Drive Smyrna, GA 30080
US License No. 1736This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Issued: 9/2024
Close -
INSTRUCTIONS for USE BIMZELX® (bim zel' ex) (bimekizumab-bkzx) injection, for subcutaneous use single-dose prefilled syringeThis Instructions for Use contains information on how to inject BIMZELX. Read this Instructions for Use before using the BIMZELX prefilled syringe and each time you get a refill. There may be new ...
This Instructions for Use contains information on how to inject BIMZELX.
Read this Instructions for Use before using the BIMZELX prefilled syringe and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or treatment.
These instructions are for 1 injection only. You may need more than 1 injection at a time depending on your prescribed dose of BIMZELX.
Important Information you Need to Know Before Injecting BIMZELX:
- Your healthcare provider should show you or a caregiver how to prepare and inject BIMZELX using the prefilled syringe for the first time. Do not inject yourself or someone else until you have been shown how to inject BIMZELX the right way. Call your healthcare provider if you have any questions.
- The BIMZELX prefilled syringe has a needle safety feature that will be activated to cover the needle after the injection is finished. The needle safety feature will help to prevent needle stick injuries to anyone who handles the prefilled syringe after injection.
- Do not share or reuse your BIMZELX prefilled syringe. You may give or get an infection.
- Do not remove the needle cap until just before you give the injection.
How should I store BIMZELX prefilled syringe?
- Store the BIMZELX prefilled syringe in the refrigerator between 36°F to 46°F (2°C to 8°C).
- BIMZELX prefilled syringes may be stored at room temperature up to 77°F (25°C) in the original carton for up to 30 days. Do not place BIMZELX prefilled syringes back in the refrigerator after they have been stored at room temperature.
- Write the date removed from the refrigerator in the space provided on the carton and throw away if it has been kept at room temperature and not used within 30 days.
- Keep the BIMZELX prefilled syringe in the original carton until ready for use to protect from light.
- Do not freeze BIMZELX.
- Do not shake BIMZELX.
- Do not use the BIMZELX prefilled syringe past the expiration date printed on the carton.
Keep BIMZELX prefilled syringes and all medicines out of the reach of children.
BIMZELX prefilled syringe parts (see Figure A):
Supplies Needed For each BIMZELX injection:
- 1 BIMZELX prefilled syringe. You may need 2 BIMZELX prefilled syringes to give the prescribed dose.
Not provided in the BIMZELX prefilled syringe carton:
- 1 alcohol swab
- 1 clean cotton ball
- 1 sharps disposal container. See, "Step 12: Dispose of (throw away) the used BIMZELX prefilled syringe" at the end of this Instructions for Use.
Setting up for your BIMZELX injection.
Step 1: Take the BIMZELX prefilled syringe carton out of the refrigerator. Do not use the BIMZELX prefilled syringe(s) if the carton seal is broken. Throw it away and get a new one.
-
Keep the BIMZELX prefilled syringe in its original carton for 30 to 45 minutes to warm to room temperature. This will help to reduce discomfort when injecting.
- Do not microwave the prefilled syringe, run hot water over it, or leave it in direct sunlight
- Do not shake the prefilled syringe.
- Do not take the cap off the prefilled syringe until you are ready to inject.
Step 2: Find a clean, flat, and well-lit work surface, like a table.
Step 3: Gather the supplies for your injection.
Step 4: Wash your hands well with soap and water and dry with a clean towel.
Step 5: Inspect the BIMZELX prefilled syringe (see Figure B):
- Remove the BIMZELX prefilled syringe from the carton.
-
Make sure the name BIMZELX appears on the label.
- Check the expiration date printed on the label.
- Check the medicine through the viewing window. The medicine inside should be clear to slightly pearly, and colorless to pale brownish-yellow. You may see air bubble(s) in the liquid. This is normal.
-
Do not use the BIMZELX prefilled syringe, throw it away and get a new one if:
- The expiration date printed on the label has passed
- The medicine is cloudy, discolored, or has particles
- It looks damaged or has been dropped
Choose and prepare your injection site.
Step 6: Choose and clean your injection site.
- The sites you may choose for your injection are:
- Do not inject into areas where the skin is tender, bruised, red, hard, thick, scaly, or affected by psoriasis, or within 2 inches of the belly button (navel).
- Choose a new injection site on the body each time you use BIMZELX. Do not use the same injection site 2 times in a row.
Step 7: Prepare your skin.
- Clean the injection site with an alcohol swab. Let the area dry completely. Do not touch the cleaned area again before injecting.
Injecting BIMZELX.
Step 8: Remove the BIMZELX prefilled syringe needle cap.
- Hold the BIMZELX prefilled syringe around the finger grip with one hand. Pull the cap straight off (away from) the BIMZELX prefilled syringe with the other hand (see Figure E). You may see a drop of liquid on the tip of the needle, this is normal.
- Put the cap into an FDA-cleared sharps disposal container right away. You will not need it again.
- Do not touch the needle or let the needle touch any surface.
- Do not hold the plunger rod during cap removal. If you accidentally remove the plunger rod, throw the BIMZELX prefilled syringe away and call your healthcare provider.
- Do not put the needle cap back on.
Step 9: Gently pinch and hold a fold of skin where you cleaned the injection site with one hand. With the other hand, insert the needle into your skin at about a 45-degree angle.
- Push the needle all the way in to make sure that you inject your full dose. Then gently let go of your skin. Make sure the needle is in place (see Figure F).
Step 10: Firmly push the plunger head all the way down until all the medicine is injected. (see Figure G).
- All the medicine is injected when you cannot push the plunger head any further (see Figure H).
Step 11: Lift your thumb off the plunger head (see Figure I). The needle will automatically move back in and lock in place.
- Press a clean cotton ball or gauze pad over the injection site for a few seconds. Do not rub the injection site. You may see slight bleeding or a drop of liquid. This is normal. You may cover the injection site with a small adhesive bandage, if needed.
Step 12: Dispose of (throw away) the used BIMZELX prefilled syringe (see Figure J).
- Put the used BIMZELX prefilled syringe in an FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) the BIMZELX prefilled syringe in your household trash. If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labeled to warn of hazardous waste inside the container.
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal.
- Do not recycle your used sharps disposal container.
Step 13: Repeat steps 1 through 12 with a new BIMZELX prefilled syringe if you need to use 2 prefilled syringes to give the prescribed dose.
- Make sure to select a new injection site on the body each time you use BIMZELX. Do not use the same site that you used for your first injection.
You can sign up to receive sharps containers for BIMZELX syringe disposal at no additional cost by going to www.BIMZELX.com or call 1-844-599-2273.
Manufactured by:
UCB, Inc.
1950 Lake Park Drive Smyrna, GA 30080
US License No. 1736This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Issued: 9/2024
Close -
INSTRUCTIONS for USEBIMZELX® (bim zel' ex)(bimekizumab-bkzx)injection, for subcutaneous use320 mg/2 mL single-dose autoinjectorThis Instructions for Use contains information on how to inject BIMZELX. Read this Instructions for Use before using the BIMZELX autoinjector and each time you get a refill. There may be new ...
This Instructions for Use contains information on how to inject BIMZELX.
Read this Instructions for Use before using the BIMZELX autoinjector and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or treatment.
Each BIMZELX autoinjector is for one-time (single-dose) use only.
Important Information you Need to Know Before Injecting BIMZELX:
- Your healthcare provider should show you or a caregiver how to prepare and inject BIMZELX using the autoinjector for the first time. Do not inject yourself or someone else until you have been shown how to inject BIMZELX the right way. Call your healthcare provider if you have any questions.
- These instructions are for 1 injection only.
- The BIMZELX autoinjector has a needle safety feature that will be activated to cover the needle after the injection is finished. The needle safety feature will help to prevent needle stick injuries to anyone who handles the autoinjector after injection.
- Do not share or reuse your BIMZELX autoinjector. You may give or get an infection.
- Do not remove the needle cap until just before you give the injection.
- If you have vision or hearing problems, do not use the BIMZELX autoinjector without help from a caregiver.
How should I store BIMZELX autoinjector?
- Store the BIMZELX autoinjector in the refrigerator between 36°F to 46°F (2°C to 8°C).
- BIMZELX autoinjectors may be stored at room temperature up to 77°F (25°C) in the original carton for up to 30 days. Do not place BIMZELX autoinjectors back in the refrigerator after they have been stored at room temperature.
- Write the date removed from the refrigerator in the space provided on the carton and throw away if it has been kept at room temperature and not used within 30 days.
- Keep BIMZELX in the original carton until ready for use to protect from light.
- Do not freeze BIMZELX.
- Do not shake BIMZELX.
- Do not use the BIMZELX autoinjector past the expiration date printed on the carton.
Keep BIMZELX autoinjectors and all medicines out of the reach of children.
BIMZELX autoinjector parts (see Figure A):
Supplies Needed for each BIMZELX injection
- 1 BIMZELX autoinjector
Not provided in the BIMZELX autoinjector carton:
- 1 alcohol swab
- 1 clean cotton ball
- 1 sharps disposal container. See, "Step 12: Dispose of (throw away) the used BIMZELX autoinjector" at the end of this Instructions for Use.
Setting up for your BIMZELX injection.
Step 1: Take the BIMZELX autoinjector carton out of the refrigerator. Do not use the BIMZELX autoinjector(s) if the carton seal is broken. Throw it away and get a new one.
-
Keep the BIMZELX autoinjector in its original carton for 30 to 45 minutes to warm to room temperature. This will help to reduce discomfort when injecting.
- Do not microwave the autoinjector, run hot water over it, or leave it in direct sunlight.
- Do not shake the autoinjector.
- Do not take the cap off the autoinjector until you are ready to inject.
Step 2: Find a clean, flat, and well-lit work surface, like a table.
Step 3: Gather the supplies needed for your injection.
Step 4: Wash your hands well with soap and water and dry with a clean towel.
Step 5: Inspect the BIMZELX autoinjector (see Figure B):
- Remove the BIMZELX autoinjector from the carton.
-
Make sure the name BIMZELX appears on the label.
- Check the expiration date printed on the label.
- Check the medicine through the Viewing Window. The medicine inside should be clear to slightly pearly, and colorless to pale brownish-yellow. You may see air bubble(s) in the liquid. This is normal.
-
Do not use the BIMZELX autoinjector, throw it away and get a new one if:
- The expiration date printed on the label has passed.
- The medicine is cloudy, discolored, or has particles.
- It looks damaged or has been dropped.
Choose and prepare your injection site.
Step 6: Choose and clean your injection site.
- The sites you may choose for your injection are:
- Do not inject into areas where the skin is tender, bruised, red, hard, thick, scaly, or affected by psoriasis, or within 2 inches of the belly button (navel).
- You should change (rotate) your injection site with each injection. Do not use the same injection site 2 times in a row.
Step 7: Prepare your skin
- Clean the injection site with an alcohol swab. Let the area dry completely. Do not touch the cleaned area again before injecting.
Injecting BIMZELX.
Step 8: Remove the BIMZELX autoinjector cap.
- Hold the BIMZELX autoinjector firmly with one hand around the handle. Pull the cap straight off (away from) the autoinjector with the other hand (see Figure E). Although you cannot see the needle tip, it is now uncovered.
- Put the cap into an FDA-cleared sharps disposal container right away. You will not need it again.
- Do not touch the needle guard or put the cap back on as it could activate the autoinjector and you can stick yourself.
Step 9: Hold the BIMZELX autoinjector at a 90-degree angle to the cleaned injection site (see Figure F).
Step 10: Place the BIMZELX autoinjector flat against your skin, then firmly press the BIMZELX autoinjector down against your skin



You will hear a "click" sound. Your injection begins when the 1st "click" is heard (see Figure G). - Do not lift the autoinjector away from the skin.
- You will hear a 2nd "click" that tells you the injection is almost complete. You should see the yellow color indicator filling the viewing window (see Figure H).
- After the 2nd click, continue to hold down the BIMZELX autoinjector for an additional 5 seconds (slowly count to 5). This will ensure you receive your full dose (see Figure I).
Important: When you remove the autoinjector, if the window has not turned yellow this means you may not have received a full dose. Call your healthcare provider right away.
Step 11: Remove the BIMZELX autoinjector by carefully pulling the BIMZELX autoinjector straight up from your skin. The needle guard will automatically cover the needle. Do not try to touch the needle.
- Press a clean cotton ball over the injection site for a few seconds. Do not rub the injection site. You may see slight bleeding or a drop of liquid. This is normal. You may cover the injection site with a small adhesive bandage, if needed.
Step 12: Dispose of (throw away) the used BIMZELX autoinjector (see Figure I).
- Put the used BIMZELX autoinjector in an FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) the BIMZELX autoinjector in your household trash. If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labeled to warn of hazardous waste inside the container.
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state you live in, go to the FDA's website at:
http://www.fda.gov/safesharpsdisposal. - Do not recycle your used sharps disposal container.
You can sign up to receive sharps containers for BIMZELX autoinjector disposal at no additional cost by going to www.BIMZELX.com or call 1-844-599-2273.
Manufactured by:
UCB, Inc. 1950 Lake Park Drive Smyrna, GA 30080
US License No. 1736This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Revised: 10/2024
Close -
INSTRUCTIONS for USEBIMZELX® (bim zel' ex)(bimekizumab-bkzx)injection, for subcutaneous use320 mg/ 2 mL single-dose prefilled syringeThis Instructions for Use contains information on how to inject BIMZELX. Read this Instructions for Use before using the BIMZELX prefilled syringe and each time you get a refill. There may be new ...
This Instructions for Use contains information on how to inject BIMZELX.
Read this Instructions for Use before using the BIMZELX prefilled syringe and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or treatment.
Each BIMZELX prefilled syringe is for one-time (single-dose) use only.
Important Information you Need to Know Before Injecting BIMZELX:
- Your healthcare provider should show you or a caregiver how to prepare and inject BIMZELX using the prefilled syringe for the first time. Do not inject yourself or someone else until you have been shown how to inject BIMZELX the right way. Call your healthcare provider if you have any questions.
- These instructions are for 1 injection only.
- The BIMZELX prefilled syringe has a needle safety feature that will be activated to cover the needle after the injection is finished. The needle safety feature will help to prevent needle stick injuries to anyone who handles the prefilled syringe after injection.
- Do not share or reuse your BIMZELX prefilled syringe. You may give or get an infection.
- Do not remove the needle cap until just before you give the injection.
How should I store BIMZELX prefilled syringe?
- Store the BIMZELX prefilled syringe in the refrigerator between 36°F to 46°F (2°C to 8°C).
- BIMZELX prefilled syringes may be stored at room temperature up to 77°F (25°C) in the original carton for up to 30 days. Do not place BIMZELX prefilled syringes back in the refrigerator after they have been stored at room temperature.
- Write the date removed from the refrigerator in the space provided on the carton and throw away if it has been kept at room temperature and not used within 30 days.
- Keep the BIMZELX prefilled syringe in the original carton until ready for use to protect from light.
- Do not freeze BIMZELX.
- Do not shake BIMZELX.
- Do not use the BIMZELX prefilled syringe past the expiration date printed on the carton.
Keep BIMZELX prefilled syringes and all medicines out of the reach of children.
BIMZELX prefilled syringe parts (see Figure A):
Supplies Needed For each BIMZELX injection:
- 1 BIMZELX prefilled syringe
Not provided in the BIMZELX prefilled syringe carton:
- 1 alcohol swab
- 1 clean cotton ball
- 1 sharps disposal container. See, "Step 12: Dispose of (throw away) the used BIMZELX prefilled syringe" at the end of this Instructions for Use.
Setting up for your BIMZELX injection.
Step 1: Take the BIMZELX prefilled syringe carton out of the refrigerator. Do not use the BIMZELX prefilled syringe(s) if the carton seal is broken. Throw it away and get a new one.
-
Keep the BIMZELX prefilled syringe in its original carton for 30 to 45 minutes to warm to room temperature. This will help to reduce discomfort when injecting.
- Do not microwave the prefilled syringe, run hot water over it, or leave it in direct sunlight
- Do not shake the prefilled syringe.
- Do not take the cap off the prefilled syringe until you are ready to inject.
Step 2: Find a clean, flat, and well-lit work surface, like a table.
Step 3: Gather the supplies for your injection.
Step 4: Wash your hands well with soap and water and dry with a clean towel.
Step 5: Inspect the BIMZELX prefilled syringe (see Figure B):
- Remove the BIMZELX prefilled syringe from the carton.
-
Make sure the name BIMZELX appears on the label.
- Check the expiration date printed on the label.
- Check the medicine through the viewing window. The medicine inside should be clear to slightly pearly, and colorless to pale brownish-yellow. You may see air bubble(s) in the liquid. This is normal.
-
Do not use the BIMZELX prefilled syringe, throw it away and get a new one if:
- The expiration date printed on the label has passed
- The medicine is cloudy, discolored, or has particles
- It looks damaged or has been dropped
Choose and prepare your injection site.
Step 6: Choose and clean your injection site.
- The sites you may choose for your injection are:
- Do not inject into areas where the skin is tender, bruised, red, hard, thick, scaly, or affected by psoriasis, or within 2 inches of the belly button (navel).
- You should change (rotate) your injection site with each injection. Do not use the same injection site 2 times in a row.
Step 7: Prepare your skin.
- Clean the injection site with an alcohol swab. Let the area dry completely. Do not touch the cleaned area again before injecting.
Injecting BIMZELX.
Step 8: Remove the BIMZELX prefilled syringe needle cap.
- Hold the BIMZELX prefilled syringe around the finger grip with one hand. Pull the cap straight off (away from) the BIMZELX prefilled syringe with the other hand (see Figure E). You may see a drop of liquid on the tip of the needle, this is normal.
- Put the cap into an FDA-cleared sharps disposal container right away. You will not need it again.
- Do not touch the needle or let the needle touch any surface.
- Do not hold the plunger rod during cap removal. If you accidentally remove the plunger rod, throw the BIMZELX prefilled syringe away and call your healthcare provider.
- Do not put the needle cap back on.
Step 9: Gently pinch and hold a fold of skin where you cleaned the injection site with one hand. With the other hand, insert the needle into your skin at about a 45-degree angle.
- Push the needle all the way in to make sure that you inject your full dose. Then gently let go of your skin. Make sure the needle is in place (see Figure F).
Step 10: Firmly push the plunger head all the way down until all the medicine is injected. (see Figure G).
- All the medicine is injected when you cannot push the plunger head any further (see Figure H).
Step 11: Lift your thumb off the plunger head (see Figure I). The needle will automatically move back in and lock in place.
- Press a clean cotton ball or gauze pad over the injection site for a few seconds. Do not rub the injection site. You may see slight bleeding or a drop of liquid. This is normal. You may cover the injection site with a small adhesive bandage, if needed.
Step 12: Dispose of (throw away) the used BIMZELX prefilled syringe (see Figure J).
- Put the used BIMZELX prefilled syringe in an FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) the BIMZELX prefilled syringe in your household trash. If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labeled to warn of hazardous waste inside the container.
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal.
- Do not recycle your used sharps disposal container.
You can sign up to receive sharps containers for BIMZELX syringe disposal at no additional cost by going to www.BIMZELX.com or call 1-844-599-2273.
Manufactured by:
UCB, Inc.
1950 Lake Park Drive Smyrna, GA 30080US License No. 1736
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Close
Revised: 10/2024 -
PRINCIPAL DISPLAY PANEL - 160 mg/mL Autoinjector CartonNDC 50474-781-85 - Rx ONLY - ATTENTION: Dispense enclosed - Medication Guide to each patient. Bimzelx® (bimekizumab-bkzx) Injection - 160 mg/mL per autoinjector - FOR SUBCUTANEOUS USE ONLY - Two ...
NDC 50474-781-85
Rx ONLYATTENTION: Dispense enclosed
Medication Guide to each patient.Bimzelx®
(bimekizumab-bkzx) Injection160 mg/mL per autoinjector
FOR SUBCUTANEOUS USE ONLYTwo single-dose autoinjectors.
Each autoinjector delivers 160 mg
in 1 mL of bimekizumab-bkzx.For a 320 mg dose, two 160 mg
autoinjectors are required.Close
-
PRINCIPAL DISPLAY PANEL - 160 mg/mL Syringe CartonNDC 50474-780-79 - Rx ONLY - ATTENTION: Dispense enclosed - Medication Guide to each patient. Bimzelx® (bimekizumab-bkzx) Injection - 160 mg/mL per syringe - FOR SUBCUTANEOUS USE ONLY - Two single-dose ...
NDC 50474-780-79
Rx ONLYATTENTION: Dispense enclosed
Medication Guide to each patient.Bimzelx®
(bimekizumab-bkzx) Injection160 mg/mL per syringe
FOR SUBCUTANEOUS USE ONLYTwo single-dose prefilled
syringes. Each syringe
delivers 160 mg in 1 mL
of bimekizumab-bkzx.For a 320 mg dose, two 160 mg
prefilled syringes are required.Close
-
PRINCIPAL DISPLAY PANEL - 320 mg/2 mL autoinjector CartonNDC 50474-782-84 - Rx ONLY - ATTENTION: Dispense enclosed - Medication Guide to each patient. Bimzelx® (bimekizumab-bkzx) Injection - 320 mg/2 mL autoinjector - FOR SUBCUTANEOUS USE ONLY - One single-dose ...
NDC 50474-782-84
Rx ONLYATTENTION: Dispense enclosed
Medication Guide to each patient.Bimzelx®
(bimekizumab-bkzx) Injection320 mg/2 mL autoinjector
FOR SUBCUTANEOUS USE ONLY
One single-dose autoinjector.
Each autoinjector delivers 320 mg
in 2 mL of bimekizumab-bkzx.Close
-
PRINCIPAL DISPLAY PANEL - 320 mg/2 mL Syringe CartonNDC 50474-783-78 - Rx ONLY - ATTENTION: Dispense enclosed - Medication Guide to each patient. Bimzelx® (bimekizumab-bkzx) Injection - 320 mg/2 mL (160 mg/mL) syringe - FOR SUBCUTANEOUS USE ONLY - One ...
NDC 50474-783-78
Rx ONLYATTENTION: Dispense enclosed
Medication Guide to each patient.Bimzelx®
(bimekizumab-bkzx) Injection320 mg/2 mL (160 mg/mL)
syringeFOR SUBCUTANEOUS USE ONLY
One single-dose prefilled syringe.
Each syringe delivers 320 mg
in 2 mL of bimekizumab-bkzx.Close
-
INGREDIENTS AND APPEARANCEProduct Information
BIMZELX bimekizumab injection, solution Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:50474-781 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength bimekizumab (UNII: 09495UIM6V) (bimekizumab - UNII:09495UIM6V) bimekizumab 160 mg in 1 mL Inactive Ingredients Ingredient Name Strength acetic acid (UNII: Q40Q9N063P) 1.23 mg in 1 mL glycine (UNII: TE7660XO1C) 16.5 mg in 1 mL polysorbate 80 (UNII: 6OZP39ZG8H) 0.4 mg in 1 mL SODIUM ACETATE (UNII: 4550K0SC9B) 2.83 mg in 1 mL WATER (UNII: 059QF0KO0R) Product Characteristics Color YELLOW (brownish to yellow) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50474-781-85 2 in 1 CARTON 10/17/2023 1 1 mL in 1 SYRINGE, GLASS; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) 2 NDC:50474-781-87 2 in 1 CARTON 10/17/2023 2 1 mL in 1 SYRINGE, GLASS; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) 3 NDC:50474-781-84 1 in 1 CARTON 10/01/2024 3 1 mL in 1 SYRINGE, GLASS; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) 4 NDC:50474-781-86 1 in 1 CARTON 10/01/2024 4 1 mL in 1 SYRINGE, GLASS; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761151 10/17/2023 BIMZELX bimekizumab injection, solution Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:50474-780 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength bimekizumab (UNII: 09495UIM6V) (bimekizumab - UNII:09495UIM6V) bimekizumab 160 mg in 1 mL Inactive Ingredients Ingredient Name Strength acetic acid (UNII: Q40Q9N063P) 1.23 mg in 1 mL glycine (UNII: TE7660XO1C) 16.5 mg in 1 mL polysorbate 80 (UNII: 6OZP39ZG8H) 0.4 mg in 1 mL SODIUM ACETATE (UNII: 4550K0SC9B) 2.83 mg in 1 mL WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50474-780-79 2 in 1 CARTON 10/17/2023 1 1 mL in 1 SYRINGE, GLASS; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) 2 NDC:50474-780-78 1 in 1 CARTON 10/01/2024 2 1 mL in 1 SYRINGE, GLASS; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761151 10/17/2023 BIMZELX bimekizumab injection, solution Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:50474-782 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength bimekizumab (UNII: 09495UIM6V) (bimekizumab - UNII:09495UIM6V) bimekizumab 320 mg in 2 mL Inactive Ingredients Ingredient Name Strength acetic acid (UNII: Q40Q9N063P) 2.46 mg in 2 mL glycine (UNII: TE7660XO1C) 33 mg in 2 mL polysorbate 80 (UNII: 6OZP39ZG8H) 0.8 mg in 2 mL SODIUM ACETATE (UNII: 4550K0SC9B) 5.65 mg in 2 mL WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50474-782-84 1 in 1 CARTON 12/05/2024 1 2 mL in 1 SYRINGE, GLASS; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) 2 NDC:50474-782-86 1 in 1 CARTON 12/05/2024 2 1 mL in 1 SYRINGE, GLASS; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761151 12/05/2024 BIMZELX bimekizumab injection, solution Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:50474-783 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength bimekizumab (UNII: 09495UIM6V) (bimekizumab - UNII:09495UIM6V) bimekizumab 320 mg in 2 mL Inactive Ingredients Ingredient Name Strength acetic acid (UNII: Q40Q9N063P) 2.46 mg in 2 mL glycine (UNII: TE7660XO1C) 33 mg in 2 mL polysorbate 80 (UNII: 6OZP39ZG8H) 0.8 mg in 2 mL SODIUM ACETATE (UNII: 4550K0SC9B) 5.65 mg in 2 mL WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50474-783-78 1 in 1 CARTON 12/05/2024 1 2 mL in 1 SYRINGE, GLASS; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761151 12/05/2024
CloseLabeler - UCB, Inc. (028526403)
Find additional resources
(also available in the left menu)Safety
Report Adverse Events, FDA Safety Recalls, Presence in Breast Milk
Related Resources
Medline Plus, Clinical Trials, PubMed, Biochemical Data Summary
More Info on this Drug
View Labeling Archives, RxNorm, Get Label RSS Feed, View NDC Code(s)NEW!
RxNorm
BIMZELX- bimekizumab injection, solution
| RxCUI | RxNorm NAME | RxTTY | |
|---|---|---|---|
| 1 | 2668086 | bimekizumab-bkzx 160 MG in 1 ML Prefilled Syringe | PSN |
| 2 | 2668086 | 1 ML bimekizumab-bkzx 160 MG/ML Prefilled Syringe | SCD |
| 3 | 2668086 | bimekizumab-bkzx 160 MG per 1 ML Prefilled Syringe | SY |
| 4 | 2668093 | Bimzelx 160 MG in 1 ML Prefilled Syringe | PSN |
| 5 | 2668093 | 1 ML bimekizumab-bkzx 160 MG/ML Prefilled Syringe [Bimzelx] | SBD |
| 6 | 2668093 | Bimzelx 160 MG per 1 ML Prefilled Syringe | SY |
| 7 | 2668099 | bimekizumab-bkzx 160 MG in 1 ML Auto-Injector | PSN |
| 8 | 2668099 | 1 ML bimekizumab-bkzx 160 MG/ML Auto-Injector | SCD |
| 9 | 2668099 | bimekizumab-bkzx 160 MG per 1 ML Auto-Injector | SY |
| 10 | 2668103 | Bimzelx 160 MG in 1 ML Auto-Injector | PSN |
| 11 | 2668103 | 1 ML bimekizumab-bkzx 160 MG/ML Auto-Injector [Bimzelx] | SBD |
| 12 | 2668103 | 1 ML Bimzelx 160 MG/ML Auto-Injector | SY |
| 13 | 2668103 | Bimzelx 160 MG per 1 ML Auto-Injector | SY |
| 14 | 2699121 | bimekizumab-bkzx 320 MG in 2 ML Auto-Injector | PSN |
| 15 | 2699121 | 2 ML bimekizumab-bkzx 160 MG/ML Auto-Injector | SCD |
| 16 | 2699121 | bimekizumab-bkzx 320 MG per 2 ML Auto-Injector | SY |
| 17 | 2699122 | Bimzelx 320 MG in 2 ML Auto-Injector | PSN |
| 18 | 2699122 | 2 ML bimekizumab-bkzx 160 MG/ML Auto-Injector [Bimzelx] | SBD |
| 19 | 2699122 | Bimzelx 320 MG per 2 ML Auto-Injector | SY |
| 20 | 2699123 | bimekizumab-bkzx 320 MG in 2 ML Prefilled Syringe | PSN |
| 21 | 2699123 | 2 ML bimekizumab-bkzx 160 MG/ML Prefilled Syringe | SCD |
| 22 | 2699123 | bimekizumab-bkzx 320 MG per 2 ML Prefilled Syringe | SY |
| 23 | 2699124 | Bimzelx 320 MG in 2 ML Prefilled Syringe | PSN |
| 24 | 2699124 | 2 ML bimekizumab-bkzx 160 MG/ML Prefilled Syringe [Bimzelx] | SBD |
| 25 | 2699124 | Bimzelx 320 MG per 2 ML Prefilled Syringe | SY |
Get Label RSS Feed for this Drug
BIMZELX- bimekizumab injection, solution
To receive this label RSS feed
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/labelrss.cfm?setid=26b88358-871f-4c80-9d80-b2fb16477f81
To receive all DailyMed Updates for the last seven days
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/rss.cfm
What will I get with the DailyMed RSS feed?
DailyMed will deliver notification of updates and additions to Drug Label information currently shown on this site through its RSS feed.
DailyMed will deliver this notification to your desktop, Web browser, or e-mail depending on the RSS Reader you select to use. To view updated drug label links, paste the RSS feed address (URL) shown below into a RSS reader, or use a browser which supports RSS feeds, such as Safari for Mac OS X.
How to discontinue the RSS feed
If you no longer wish to have this DailyMed RSS service, simply delete the copied URL from your RSS Reader.
More about getting RSS News & Updates from DailyMedWhy is DailyMed no longer displaying pill images on the Search Results and Drug Info pages?
Due to inconsistencies between the drug labels on DailyMed and the pill images provided by RxImage, we no longer display the RxImage pill images associated with drug labels.
We anticipate reposting the images once we are able identify and filter out images that do not match the information provided in the drug labels.
NDC Codes
BIMZELX- bimekizumab injection, solution
If this SPL contains inactivated NDCs listed by the FDA initiated compliance action, they will be specified as such.
| NDC | |
|---|---|
| 1 | 50474-780-78 |
| 2 | 50474-780-79 |
| 3 | 50474-781-84 |
| 4 | 50474-781-85 |
| 5 | 50474-781-86 |
| 6 | 50474-781-87 |
| 7 | 50474-782-84 |
| 8 | 50474-782-86 |
| 9 | 50474-783-78 |