Label: PIMOZIDE tablet
- NDC Code(s): 49884-347-01, 49884-348-01
- Packager: Endo USA, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 25, 2017
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Pimozide is an orally active antipsychotic agent of the diphenyl-butylpiperidine series. The structural formula of pimozide, 1-[1-[4,4-bis(4-fluorophenyl)butyl]-4-piperidinyl]-1,3-dihydro-2H-benzimidazole-2-one is:
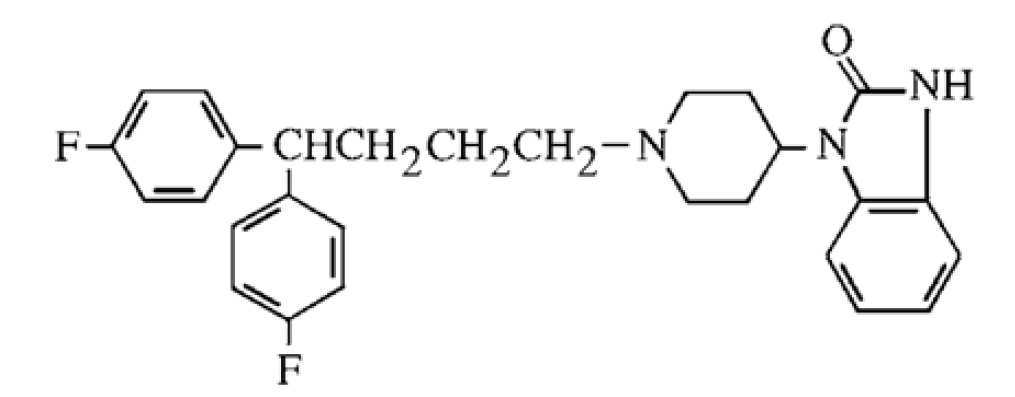
The solubility of pimozide in water is less than 0.01 mg/mL; it is slightly soluble in most organic solvents.
Each white pimozide tablet contains either 1 mg or 2 mg of pimozide and the following inactive ingredients: calcium stearate, microcrystalline cellulose, lactose anhydrous and corn starch.
-
CLINICAL PHARMACOLOGY
Pharmacodynamic Actions
Pimozide tablets, are an orally active antipsychotic drug product which shares with other antipsychotics the ability to blockade dopaminergic receptors on neurons in the central nervous system. Although its exact mode of action has not been established, the ability of pimozide to suppress motor and phonic tics in Tourette’s Disorder is thought to be a function of its dopaminergic blocking activity. However, receptor blockade is often accompanied by a series of secondary alterations in central dopamine metabolism and function which may contribute to both pimozide’s therapeutic and untoward effects. In addition, pimozide tablets, in common with other antipsychotic drugs, has various effects on other central nervous system receptor systems which are not fully characterized.Metabolism and Pharmacokinetics
More than 50% of a dose of pimozide is absorbed after oral administration. Based on the pharmacokinetic and metabolic profile, pimozide appears to undergo significant first pass metabolism. Peak serum levels occur generally six to eight hours (range 4 to 12 hours) after dosing.
Pimozide is extensively metabolized, primarily by N-dealkylation in the liver. This metabolism is catalyzed mainly by the cytochrome P450 3A4 (CYP 3A4) enzymatic system and to a lesser extent, by cytochrome P450 1A2 (CYP 1A2) and cytochrome P450 2D6 (CYP 2D6). Two major metabolites have been identified, 1-(4-piperidyl)-2-benzimidazolinone and 4,4-bis(4-fluorophenyl) butyric acid. The antipsychotic activity of these metabolites is undetermined. The major route of elimination of pimozide and its metabolites is through the kidney.
The mean serum elimination half-life of pimozide in schizophrenic patients was approximately 55 hours. There was a 13-fold interindividual difference in the area under the serum pimozide level-time curve and an equivalent degree of variation in peak serum levels among patients studied. The significance of this is unclear since there are few correlations between plasma levels and clinical findings.
Effects of food and disease upon the absorption, distribution, metabolism and elimination of pimozide are not known. Effects of concomitant medication and genetic variations on pimozide metabolism are described in the CONTRAINDICATIONS and PRECAUTIONS sections.
-
INDICATIONS AND USAGE
Pimozide tablets, USP are indicated for the suppression of motor and phonic tics in patients with Tourette’s Disorder who have failed to respond satisfactorily to standard treatment. Pimozide Tablets, USP are not intended as a treatment of first choice nor is it intended for the treatment of tics that are merely annoying or cosmetically troublesome. Pimozide Tablets, USP should be reserved for use in Tourette’s Disorder patients whose development and/or daily life function is severely compromised by the presence of motor and phonic tics.
Evidence supporting approval of pimozide tablets, USP for use in Tourette’s Disorder was obtained in two controlled clinical investigations which enrolled patients between the ages of 8 and 53 years. Most subjects in the two trials were 12 or older.
-
CONTRAINDICATIONS
1. Pimozide is contraindicated in the treatment of simple tics or tics other than those associated with Tourette’s Disorder.
2. Pimozide should not be used in patients taking drugs that may, themselves, cause motor and phonic tics (e.g., pemoline, methylphenidate and amphetamines) until such patients have been withdrawn from these drugs to determine whether or not the drugs, rather than Tourette’s Disorder, are responsible for the tics.
3. Because pimozide prolongs the QT interval of the electrocardiogram it is contraindicated in patients with congenital long QT syndrome, patients with a history of cardiac arrhythmias, patients taking other drugs which prolong the QT interval of the electrocardiogram or patients with known hypokalemia or hypomagnesemia (see also PRECAUTIONS - DRUG INTERACTIONS).
4. Pimozide is contraindicated in patients with severe toxic central nervous system depression or comatose states from any cause.
5. Pimozide is contraindicated in patients with hypersensitivity to it. As it is not known whether cross-sensitivity exists among the antipsychotics, pimozide should be used with appropriate caution in patients who have demonstrated hypersensitivity to other antipsychotic drugs.
6. Ventricular arrhythmias have been rarely associated with the use of macrolide antibiotics in patients with prolonged QT intervals, as might be produced by pimozide. Specifically, two sudden deaths have been reported when clarithromycin was added to ongoing pimozide therapy. Furthermore, some evidence suggests that pimozide is metabolized partly by the enzyme system cytochrome P450 3A4 (CYP 3A4). Macrolide antibiotics are inhibitors of CYP 3A4, and thus could potentially impede pimozide metabolism. For these reasons, pimozide is contraindicated in patients receiving the macrolide antibiotics clarithromycin, erythromycin, azithromycin, dirithromycin, and troleandomycin.
7. Concomitant use in patients taking Celexa or Lexapro is contraindicated (see PRECAUTIONS - DRUG INTERACTIONS - Pimozide and Celexa).
8. Clinical drug interaction studies have demonstrated that pimozide is also metabolized by CYP 2D6. Concomitant use of pimozide with paroxetine and other strong CYP 2D6 inhibitors is contraindicated (See PRECAUTIONS – DRUG INTERACTIONS).
9. Concomitant use of pimozide in patients taking sertraline is contraindicated (See PRECAUTIONS – DRUG INTERACTIONS).
Because azole antifungal agents are also inhibitors of the CYP 3A4 enzymes and thus may likewise impair pimozide metabolism, pimozide is contraindicated in patients receiving the azole antifungal agents itraconazole and ketoconazole.
Similarly, protease inhibitor drugs are also inhibitors of CYP 3A4, and thus pimozide is contraindicated in patients receiving protease inhibitors such as ritonavir, saquinovir, indinavir, and nelfinavir. (See PRECAUTIONS - DRUG INTERACTIONS.)
Nefazodone is a potent inhibitor of CYP 3A4, and its concomitant use with pimozide is also contraindicated.
Other drugs that are relatively less potent inhibitors of CYP 3A4 should also be avoided, in view of the risks: e.g., zileuton, fluvoxamine.
-
WARNINGS
The use of pimozide in the treatment of Tourette’s Disorder involves different risk/benefit considerations than when antipsychotic drugs are used to treat other conditions. Consequently, a decision to use pimozide should take into consideration the following (see also PRECAUTIONS - Information for Patients).
Tardive Dyskinesia
A syndrome consisting of potentially irreversible, involuntary, dyskinetic movements may develop in patients treated with antipsychotic drugs. Although the prevalence of the syndrome appears to be highest among the elderly, especially elderly women, it is impossible to rely upon prevalence estimates to predict, at the inception of antipsychotic treatment, which patients are likely to develop the syndrome. Whether antipsychotic drug products differ in their potential to cause tardive dyskinesia is unknown.
Both the risk of developing tardive dyskinesia and the likelihood that it will become irreversible are believed to increase as the duration of treatment and the total cumulative dose of antipsychotic drugs administered to the patient increase. However, the syndrome can develop, although much less commonly, after relatively brief treatment periods at low doses.
There is no known treatment for established cases of tardive dyskinesia, although the syndrome may remit, partially or completely, if antipsychotic treatment is withdrawn. Antipsychotic treatment itself, however, may suppress (or partially suppress) the signs and symptoms of the syndrome and thereby may possibly mask the underlying process. The effect that symptomatic suppression has upon the long-term course of the syndrome is unknown.
Given these considerations, antipsychotic drugs should be prescribed in a manner that is most likely to minimize the occurrence of tardive dyskinesia. Chronic antipsychotic treatment should generally be reserved for patients who suffer from a chronic illness, that 1) is known to respond to antipsychotic drugs, and, 2) for whom alternative, equally effective, but potentially less harmful treatments are not available or appropriate. In patients who do require chronic treatment, the smallest dose and the shortest duration of treatment producing a satisfactory clinical response should be sought. The need for continued treatment should be reassessed periodically.
If signs and symptoms of tardive dyskinesia appear in a patient on antipsychotics, drug discontinuation should be considered. However, some patients may require treatment despite the presence of the syndrome.
(For further information about the description of tardive dyskinesia and its clinical detection, please refer to ADVERSE REACTIONS and PRECAUTIONS - Information for Patients.)
Neuroleptic Malignant Syndrome (NMS)
A potentially fatal symptom complex sometimes referred to as Neuroleptic Malignant Syndrome (NMS) has been reported in association with antipsychotic drugs. Clinical manifestations of NMS are hyperpyrexia, muscle rigidity, altered mental status (including catatonic signs) and evidence of autonomic instability (irregular pulse or blood pressure, tachycardia, diaphoresis, and cardiac dysrhythmias). Additional signs may include elevated creatine phosphokinase, myoglobinuria (rhabdomyolysis) and acute renal failure.
The diagnostic evaluation of patients with this syndrome is complicated. In arriving at a diagnosis, it is important to identify cases where the clinical presentation includes both serious medical illness (e.g., pneumonia, systemic infection, etc.) and untreated or inadequately treated extrapyramidal signs and symptoms (EPS). Other important considerations in the differential diagnosis include central anticholinergic toxicity, heat stroke, drug fever and primary central nervous system (CNS) pathology.
The management of NMS should include 1) immediate discontinuation of antipsychotic drugs and other drugs not essential to concurrent therapy, 2) intensive symptomatic treatment and medical monitoring, and 3) treatment of any concomitant serious medical problems for which specific treatments are available. There is no general agreement about specific pharmacological treatment regimens for uncomplicated NMS.
If a patient requires antipsychotic drug treatment after recovery from NMS, the potential reintroduction of drug therapy should be carefully considered. The patient should be carefully monitored, since recurrences of NMS have been reported.
Hyperpyrexia, not associated with the above symptom complex, has been reported with other antipsychotic drugs.
Other
Sudden, unexpected deaths have occurred in experimental studies of conditions other than Tourette’s Disorder. These deaths occurred while patients were receiving dosages in the range of 1 mg per kg. One possible mechanism for such deaths is prolongation of the QT interval predisposing patients to ventricular arrhythmia. An electrocardiogram should be performed before pimozide treatment is initiated and periodically thereafter, especially during the period of dose adjustment.
Pimozide may have a tumorigenic potential. Based on studies conducted in mice, it is known that pimozide can produce a dose-related increase in pituitary tumors. The full significance of this finding is not known, but should be taken into consideration in the physician’s and patient’s decisions to use this drug product. This finding should be given special consideration when the patient is young and chronic use of pimozide is anticipated (see PRECAUTIONS - Carcinogenesis, Mutagenesis, Impairment of Fertility).
-
PRECAUTIONS
Leukopenia, Neutropenia and Agranulocytosis
Class Effect: In clinical trial and/or postmarketing experience, events of leukopenia/neutropenia and agranulocytosis have been reported temporally related to antipsychotic agents.
Possible risk factors for leukopenia/neutropenia include preexisting low white blood cell count (WBC) and history of drug induced leukopenia/neutropenia. Patients with a history of a clinically significant low WBC or drug induced leukopenia/neutropenia should have their complete blood count (CBC) monitored frequently during the first few months of therapy and discontinuation of pimozide should be considered at the first sign of a clinically significant decline in WBC in the absence of other causative factors.
Patients with clinically significant neutropenia should be carefully monitored for fever or other symptoms or signs of infection and treated promptly if such symptoms or signs occur. Patients with severe neutropenia (absolute neutrophil count <1000/mm3) should discontinue pimozide and have their WBC followed until recovery.
General
Pimozide may impair the mental and/or physical abilities required for the performance of potentially hazardous tasks, such as driving a car or operating machinery, especially during the first few days of therapy.
Pimozide produces anticholinergic side effects and should be used with caution in individuals whose conditions may be aggravated by anticholinergic activity.
Pimozide should be administered cautiously to patients with impairment of liver or kidney function, because it is metabolized by the liver and excreted by the kidneys.
Antipsychotics should be administered with caution to patients receiving anticonvulsant medication, with a history of seizures, or with EEG abnormalities, because they may lower the convulsive threshold. If indicated, adequate anticonvulsant therapy should be maintained concomitantly.Information for Patients
Treatment with pimozide tablets, USP exposes the patient to serious risks. A decision to use pimozide tablets, USP chronically in Tourette’s Disorder is one that deserves full consideration by the patient (or patient’s family) as well as by the treating physician. Because the goal of treatment is symptomatic improvement, the patient’s view of the need for treatment and assessment of response are critical in evaluating the impact of therapy and weighing its benefits against the risks. Since the physician is the primary source of information about the use of a drug in any disease, it is recommended that the following information be discussed with patients and/or their families.
Pimozide tablets, are intended only for use in patients with Tourette’s Disorder whose symptoms are severe and who cannot tolerate, or who do not respond to HALDOL® (haloperidol).
Given the likelihood that a proportion of patients exposed chronically to antipsychotics will develop tardive dyskinesia, it is advised that all patients in whom chronic use is contemplated be given, if possible, full information about this risk. The decision to inform patients and/or their guardians must obviously take into account the clinical circumstances and the competency of the patient to understand the information provided.
There is limited information available on the use of pimozide tablets, USP in children under 12 years of age.
The information available on pimozide tablets, USP from foreign marketing experience and from U.S. clinical trials indicates that pimozide tablets, have a side effect profile similar to that of other antipsychotic drugs. Patients should be informed that all types of side effects associated with the use of antipsychotics may be associated with the use of pimozide tablets.
In addition, sudden, unexpected deaths have occurred in patients taking high doses of pimozide tablets, USP for conditions other than Tourette’s Disorder. These deaths may have been the result of an effect of pimozide upon the heart. Therefore, patients should be instructed not to exceed the prescribed dose of pimozide tablets, USP and they should realize the need for the initial ECG and for follow-up ECGs during treatment.
Also, pimozide, at a dose about 15 times that given humans, caused an increase in the number of benign tumors of the pituitary gland in female mice. It is not possible to say how important this is. Similar tumors were not seen in rats given pimozide, nor at lower doses in mice, which is reassuring. However, any such finding must be considered to suggest a possible risk of long term use of the drug.
Because substances in grapefruit juice may inhibit the metabolism of pimozide by CYP 3A4, patients should be advised to avoid grapefruit juice.Laboratory Tests
An ECG should be done at baseline and periodically thereafter throughout the period of dose adjustment. Any indication of prolongation of QTc interval beyond an absolute limit of 0.47 seconds (children) or 0.52 seconds (adults), or more than 25% above the patient’s original baseline should be considered a basis for stopping further dose increase (see CONTRAINDICATIONS) and considering a lower dose.
Since hypokalemia has been associated with ventricular arrhythmias, potassium insufficiency, secondary to diuretics, diarrhea, or other cause, should be corrected before pimozide therapy is initiated and normal potassium maintained during therapy.
DRUG INTERACTIONS
Because pimozide prolongs the QT interval of the electrocardiogram, an additive effect on QT interval would be anticipated if administered with other drugs, such as phenothiazines, tricyclic antidepressants or antiarrhythmic agents, which prolong the QT interval. Accordingly, pimozide should not be given with dofetilide, sotalol, quinidine, other Class Ia and III anti-arrhythmics, mesoridazine, thioridazine, chlorpromazine, droperidol, sparfloxacin, gatifloxacin, moxifloxacin, halofantrine, mefloquine, pentamidine, arsenic trioxide, levomethadyl acetate, dolasetron mesylate, probucol, tacrolimus, ziprasidone, or other drugs that have demonstrated QT prolongation as one of their pharmacodynamic effects. Also, the use of macrolide antibiotics in patients with prolonged QT intervals has been rarely associated with ventricular arrhythmias. Such concomitant administration should not be undertaken (see CONTRAINDICATIONS).
Since pimozide is partly metabolized via CYP 3A4, it should not be administered concomitantly with inhibitors of this metabolic system, such as azole antifungal agents and protease inhibitor drugs (see CONTRAINDICATIONS).
Pimozide and Celexa: In a controlled study, a single dose of pimozide 2 mg coadministered with racemic citalopram 40 mg given once daily for 11 days was associated with a mean increase in QTc values of approximately 10 msec compared to pimozide given alone. Racemic citalopram did not alter the mean AUC or Cmax of pimozide. The mechanism of this pharmacodynamic interaction is not known. Concomitant use of Pimozide and Celexa or Lexapro is contraindicated (See CONTRAINDICATIONS).
CYP 2D6 inhibitors: In healthy subjects, co-administration of pimozide 2 mg (single dose) and paroxetine 60 mg resulted in a 151% increase in pimozide AUC and a 62% increase in pimozide Cmax compared to pimozide administered alone. The increase in pimozide AUC and Cmax is related to the CYP 2D6 inhibitory properties of paroxetine. Concomitant use of pimozide and paroxetine or other strong CYP 2D6 inhibitors are contraindicated (see CONTRAINDICATIONS).
As CYP 1A2 may also contribute to the metabolism of pimozide, prescribers should be aware of the theoretical potential for drug interactions with inhibitors of this enzymatic system.
Pimozide may be capable of potentiating CNS depressants, including analgesics, sedatives, anxiolytics, and alcohol.
Rare case reports have suggested possible additive effects of pimozide and fluoxetine leading to bradycardia.
Concomitant administration of pimozide and sertraline should be contraindicated (See CONTRAINDICATIONS).
Pharmacogenomics Individuals with genetic variations resulting in poor CYP 2D6 metabolism (approximately 5 to 10% of the population) exhibit higher pimozide concentrations than extensive CYP 2D6 metabolizers. The concentrations observed in poor CYP 2D6 metabolizers are similar to those seen with strong CYP 2D6 inhibitors such as paroxetine. The time to achieve steady-state pimozide concentrations is expected to be longer (approximately 2 weeks) in poor CYP 2D6 metabolizers because of the prolonged half-life. Alternative dosing strategies are recommended in patients who are genetically poor CYP 2D6 metabolizers (see DOSAGE and ADMINISTRATION).
Interaction with Food
Patients should avoid grapefruit juice because it may inhibit the metabolism of pimozide by CYP 3A4.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies were conducted in mice and rats. In mice, pimozide causes a dose-related increase in pituitary and mammary tumors.
When mice were treated for up to 18 months with pimozide, pituitary gland changes developed in females only. These changes were characterized as hyperplasia at doses approximating the human dose and adenoma at doses about fifteen times the maximum recommended human dose on a mg per kg basis. The mechanism for the induction of pituitary tumors in mice is not known.
Mammary gland tumors in female mice were also increased, but these tumors are expected in rodents treated with antipsychotic drugs which elevate prolactin levels. Chronic administration of an antipsychotic also causes elevated prolactin levels in humans. Tissue culture experiments indicate that approximately one-third of human breast cancers are prolactin-dependent in vitro, a factor of potential importance if the prescription of these drugs is contemplated in a patient with a previously detected breast cancer. Although disturbances such as galactorrhea, amenorrhea, gynecomastia, and impotence have been reported with antipsychotic drugs, the clinical significance of elevated serum prolactin levels is unknown for most patients. Neither clinical studies nor epidemiologic studies conducted to date have shown an association between chronic administration of these drugs and mammary tumorigenesis. The available evidence, however, is considered too limited to be conclusive at this time.
In a 24-month carcinogenicity study in rats, animals received up to 50 times the maximum recommended human dose. No increased incidence of overall tumors or tumors at any site was observed in either sex. Because of the limited number of animals surviving this study, the meaning of these results is unclear.
Pimozide did not have mutagenic activity in the Ames test with four bacterial test strains, in the mouse dominant lethal test or in the micronucleus test in rats.
Reproduction studies in animals were not adequate to assess all aspects of fertility. Nevertheless, female rats administered pimozide had prolonged estrus cycles, an effect also produced by other antipsychotic drugs.
Pregnancy
Teratogenic Effects: Reproduction studies performed in rats and rabbits at oral doses up to 8 times the maximum human dose did not reveal evidence of teratogenicity. In the rat, however, this multiple of the human dose resulted in decreased pregnancies and in the retarded development of fetuses. These effects are thought to be due to an inhibition or delay in implantation which is also observed in rodents administered other antipsychotic drugs. In the rabbit, maternal toxicity, mortality, decreased weight gain, and embryotoxicity including increased resorptions were dose-related. Because animal reproduction studies are not always predictive of human response, pimozide should be given to a pregnant woman only if the potential benefits of treatment clearly outweigh the potential risks.
Nonteratogenic effects. Neonates exposed to antipsychotic drugs, during the third trimester of pregnancy are at risk for extrapyramidal and/or withdrawal symptoms following delivery. There have been reports of agitation, hypertonia, hypotonia, tremor, somnolence, respiratory distress and feeding disorder in these neonates. These complications have varied in severity; while in some cases symptoms have been self-limited, in other cases neonates have required intensive care unit support and prolonged hospitalization.
Pimozide should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers
It is not known whether pimozide is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for tumorigenicity and unknown cardiovascular effects in the infant, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
Although Tourette's Disorder most often has its onset between the ages of 2 and 15 years, information on the use and efficacy of pimozide in patients less than 12 years of age is limited. A 24-week open label study in 36 children between the ages of 2 and 12 demonstrated that pimozide has a similar safety profile in this age group as in older patients and there were no safety findings that would preclude its use in this age group.
Because its use and safety have not been evaluated in other childhood disorders, pimozide is not recommended for use in any condition other than Tourette’s Disorder. -
ADVERSE REACTIONS
General
Extrapyramidal Reactions: Neuromuscular (extrapyramidal) reactions during the administration of pimozide have been reported frequently, often during the first few days of treatment. In most patients, these reactions involved Parkinson-like symptoms which, when first observed, were usually mild to moderately severe and usually reversible.
Other types of neuromuscular reactions (motor restlessness, dystonia, akathisia, hyperreflexia, opisthotonos, oculogyric crises) have been reported far less frequently. Severe extrapyramidal reactions have been reported to occur at relatively low doses. Generally the occurrence and severity of most extrapyramidal symptoms are dose-related since they occur at relatively high doses and have been shown to disappear or become less severe when the dose is reduced. Administration of antiparkinson drugs such as benztropine mesylate or trihexyphenidyl hydrochloride may be required for control of such reactions. It should be noted that persistent extrapyramidal reactions have been reported and that the drug may have to be discontinued in such cases.
Withdrawal Emergent Neurological Signs: Generally, patients receiving short term therapy experience no problems with abrupt discontinuation of antipsychotic drugs.
However, some patients on maintenance treatment experience transient dyskinetic signs after abrupt withdrawal. In certain of these cases the dyskinetic movements are indistinguishable from the syndrome described below under “Tardive Dyskinesia” except for duration. It is not known whether gradual withdrawal of antipsychotic drugs will reduce the rate of occurrence of withdrawal emergent neurological signs, but until further evidence becomes available, it seems reasonable to gradually withdraw use of pimozide.
Tardive Dyskinesia: Pimozide may be associated with persistent dyskinesias. Tardive dyskinesia, a syndrome consisting of potentially irreversible, involuntary, dyskinetic movements, may appear in some patients on long-term therapy or may occur after drug therapy has been discontinued. The risk appears to be greater in elderly patients on high-dose therapy, especially females. The symptoms are persistent and in some patients appear irreversible. The syndrome is characterized by rhythmical involuntary movements of tongue, face, mouth or jaw (e.g., protrusion of tongue, puffing of cheeks, puckering of mouth, chewing movements). Sometimes these may be accompanied by involuntary movements of extremities and the trunk.
There is no known effective treatment for tardive dyskinesia; antiparkinson agents usually do not alleviate the symptoms of this syndrome. It is suggested that all antipsychotic agents be discontinued if these symptoms appear. Should it be necessary to reinstitute treatment, or increase the dosage of the agent, or switch to a different antipsychotic agent, this syndrome may be masked.
It has been reported that fine vermicular movement of the tongue may be an early sign of tardive dyskinesia and if the medication is stopped at that time the syndrome may not develop.
Electrocardiographic Changes: Electrocardiographic changes have been observed in clinical trials of pimozide in Tourette’s Disorder and schizophrenia. These have included prolongation of the QT interval, flattening, notching and inversion of the T wave and the appearance of U waves. Sudden, unexpected deaths and grand mal seizure have occurred at doses above 20 mg/day.
Neuroleptic Malignant Syndrome: Neuroleptic malignant syndrome (NMS) has been reported with pimozide. (See WARNINGS for further information concerning NMS.)Hyperpyrexia: Hyperpyrexia has been reported with other antipsychotic drugs.
CLINICAL TRIALS
The following adverse reaction tabulation was derived from 20 patients in a 6-week long placebo-controlled clinical trial of pimozide in Tourette’s Disorder.
Body System/
Adverse Reaction
Pimozide
(N=20)
Placebo
(N=20)
Body as a Whole
Headache
1
2
Gastrointestinal
Dry Mouth
5
1
Diarrhea
1
0
Nausea
0
2
Vomiting
0
1
Constipation
4
2
Eructations
0
1
Thirsty
1
0
Appetite increase
1
0
Endocrine
Menstrual disorder
0
1
Breast secretions
0
1
Musculoskeletal
Muscle cramps
0
1
Muscle tightness
3
0
Stooped posture
2
0
CNS
Drowsiness
7
3
Sedation
14
5
Insomnia
2
2
Dizziness
0
1
Akathisia
8
0
Rigidity
2
0
Speech disorder
2
0
Handwriting change
1
0
Akinesia
8
0
Psychiatric
Depression
2
3
Excitement
0
1
Nervous
1
0
Adverse behavior effect
5
0
Special Senses
Visual disturbance
4
0
Taste change
1
0
Sensitivity of eyes to light
1
0
Decrease accommodation
4
1
Spots before eyes
0
1
Urogenital
Impotence
3
0
The following adverse event tabulation was derived from 36 children (age 2 to 12) in a 24-week open trial of pimozide in Tourette’s Disorder. Because clinical investigational experience with pimozide in Tourette’s Disorder is limited, uncommon
Body System/
Adverse Reaction
Number of Patients Experiencing Each Event (%)
All Events
(N=36)
Drug-Related
Events
(N=36)
Body as a Whole
Asthenia
9 (25.0)
5 (13.8)
Headache
8 (22.2)
1 (2.7)
Gastrointestinal
Dysphagia
1 (2.7)
1 (2.7)
Increased Salivation
5 (13.8)
2 (5.5)
Musculoskeletal
Myalgia
1 (2.7)
1 (2.7)
Central Nervous System
Dreaming Abnormal
1 (2.7)
1 (2.7)
Hyperkinesia
2 (5.5)
1 (2.7)
Somnolence
10 (27.7)
9 (25.0)
Torticollis
1 (2.7)
1 (2.7)
Tremor, Limbs
1 (2.7)
1 (2.7)
Psychiatric
Adverse Behavior Effect
10 (27.7)
8 (22.2)
Nervous
3 (8.3)
2 (5.5)
Skin
Rash
3 (8.3)
1 (2.7)
Special Senses
Visual Disturbance
2 (5.5)
1 (2.7)
Cardiovascular
ECG Abnormal
1 (2.7)
1 (2.7)
adverse reactions may not have been detected. The physician should consider that other adverse reactions associated with antipsychotics may occur.
Other Adverse Reactions
In addition to the adverse reactions listed above, those listed below have been reported in U.S. clinical trials of pimozide in conditions other than Tourette’s Disorder.
Body as a Whole: Asthenia, chest pain, periorbital edema
Cardiovascular/Respiratory: Postural hypotension, hypotension, hypertension, tachycardia, palpitationsGastrointestinal: Increased salivation, nausea, vomiting, anorexia, GI distress
Endocrine: Loss of libido
Metabolic/Nutritional: Weight gain, weight loss
Central Nervous System: Dizziness, tremor, parkinsonism, fainting, dyskinesia
Psychiatric: Excitement
Skin: Rash, sweating, skin irritation
Special Senses: Blurred vision, cataracts
Urogenital: Nocturia, urinary frequency
Postmarketing Reports
The following experiences were described in spontaneous postmarketing reports. These reports do not provide sufficient information to establish a clear causal relationship with the use of pimozide.
Gastrointestinal: Gingival hyperplasia in one patient
Hematologic: Hemolytic anemia
Metabolic/Nutritional: Hyponatremia
Other: Seizure
-
OVERDOSAGE
In general, the signs and symptoms of overdosage with pimozide would be an exaggeration of known pharmacologic effects and adverse reactions, the most prominent of which would be: 1) electrocardiographic abnormalities, 2) severe extrapyramidal reactions, 3) hypotension, 4) a comatose state with respiratory depression.
In the event of overdosage, gastric lavage, establishment of a patent airway and, if necessary, mechanically-assisted respiration are advised. Electrocardiographic monitoring should commence immediately and continue until the ECG parameters are within the normal range. Hypotension and circulatory collapse may be counteracted by use of intravenous fluids, plasma, or concentrated albumin, and vasopressor agents such as metaraminol, phenylephrine and norepinephrine.
Epinephrine should not be used. In case of severe extrapyramidal reactions, antiparkinson medication should be administered. Because of the long half-life of pimozide, patients who take an overdose should be observed for at least 4 days. As with all drugs, the physician should consider contacting a poison control center for additional information on the treatment of overdose. -
DOSAGE AND ADMINISTRATION
General
The suppression of tics by pimozide tablets, USP requires a slow and gradual introduction of the drug. The patient’s dose should be carefully adjusted to a point where the suppression of tics and the relief afforded is balanced against the untoward side effects of the drug.An ECG should be done at baseline and periodically thereafter, especially during the period of dose adjustment (see WARNINGS and PRECAUTIONS - Laboratory Tests). Periodic attempts should be made to reduce the dosage of pimozide tablets, USP to see whether or not tics persist at the level and extent first identified. In attempts to reduce the dosage of pimozide tablets, USP consideration should be given to the possibility that increases of tic intensity and frequency may represent a transient, withdrawal related phenomenon rather than a return of disease symptoms. Specifically, one to two weeks should be allowed to elapse before one concludes that an increase in tic manifestations is a function of the underlying disease syndrome rather than a response to drug withdrawal. A gradual withdrawal is recommended in any case.
Children
Reliable dose response data for the effects of pimozide tablets, USP on tic manifestation in Tourette’s Disorder patients below the age of twelve are not available.Treatment should be initiated at a dose of 0.05 mg/kg preferably taken once at bedtime. The dose may be increased every third day to a maximum of 0.2 mg/kg not to exceed 10 mg/day. At doses above 0.05 mg/kg/day, CYP 2D6 genotyping should be performed. In poor CYP 2D6 metabolizers, pimozide tablets, USP doses should not exceed 0.05 mg/kg/day, and doses should not be increased earlier than 14 days (see PRECAUTIONS – Pharmacogenomics).
Adults
In general, treatment with pimozide tablets, USP should be initiated with a dose of 1 to 2 mg a day in divided doses. The dose may be increased thereafter every other day. Most patients are maintained at less than 0.2 mg/kg/day, or 10 mg/day, whichever is less. Doses greater than 0.2 mg/kg/day or 10 mg/day are not recommended.At doses above 4 mg/day, CYP 2D6 genotyping should be performed. In poor CYP 2D6 metabolizers, pimozide tablets, USP doses should not exceed 4 mg/day, and doses should not be increased earlier than 14 days (see PRECAUTIONS – Pharmacogenomics).
- ANIMAL PHARMACOLOGY
-
HOW SUPPLIED
Pimozide Tablets USP, 1 mg are white to off-white, uncoated round shaped tablets with debossed bisect separating "EP" and "320" on one side and other side has a single bisect. They are available in bottles of 100 (NDC 49884-347-01).
Pimozide Tablets USP, 2 mg are white to off-white, uncoated oval shaped tablets with debossed bisect separating "EP" and "321" on one side and other side has a single bisect. They are available in bottles of 100 (NDC 49884-348-01).
Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature].
Pharmacist: Dispense in a tight, light-resistant container as defined in the official compendium with a child-resistant closure as required.
Rx Only
Manufactured for:
Endo USA
Malvern, PA 19355 U.S.A.
Made in India
Neutral Code: TN/DRUGS/TN00002121
© 2024 Endo, Inc. or one of its affiliates.
Revised: 08/2024 - PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PIMOZIDE
pimozide tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:49884-347 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PIMOZIDE (UNII: 1HIZ4DL86F) (PIMOZIDE - UNII:1HIZ4DL86F) PIMOZIDE 1 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) CALCIUM STEARATE (UNII: 776XM7047L) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) STARCH, CORN (UNII: O8232NY3SJ) Product Characteristics Color white Score 2 pieces Shape ROUND Size 6mm Flavor Imprint Code EP;320 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49884-347-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 09/28/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA204521 09/28/2015 PIMOZIDE
pimozide tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:49884-348 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PIMOZIDE (UNII: 1HIZ4DL86F) (PIMOZIDE - UNII:1HIZ4DL86F) PIMOZIDE 2 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) CALCIUM STEARATE (UNII: 776XM7047L) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) Product Characteristics Color white Score 2 pieces Shape OVAL Size 9mm Flavor Imprint Code EP;321 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49884-348-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 09/28/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA204521 09/28/2015 Labeler - Endo USA, Inc. (119185057)




