Label: saluron- hydroflumenthiazide tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 54092-055-01 - Packager: Shire
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated July 19, 2006
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- N/A - Section Title Not Found In Database
-
Description
Saluron® (hydroflumethiazide) is a potent oral diuretic-antihypertensive agent of low toxicity. Each tablet contains 50 mg of hydroflumethiazide.
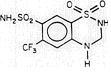
Saluron® is 2H-1,2,4-Benzothiadiazine-7-sulfonamide, 3,4-dihydro-6-(trifluoromethyl)-, 1,1-dioxide. Hydroflumethiazide is very slightly soluble in water, soluble in methanol and freely soluble in acetone. Inactive ingredients: microcrystalline cellulose, lactose, magnesium stearate, colloidal silicon dioxide, and sodium starch glycolate. It has the following structural formula:
-
Clinical Pharmacology
Hydroflumethiazide is incompletely but fairly rapidly absorbed from the gastrointestinal tract. It appears to have a biphasic biological half-life with an estimated alpha-phase of about 2 hours and an estimated beta-phase of about 17 hours; it has a metabolite with a longer half-life, which is extensively bound to the red blood cells. Hydroflumethiazide is excreted in the urine; its metabolite has also been detected in the urine.
The mechanism of action results in an interference with the renal tubular mechanism of electrolyte reabsorption. At maximal therapeutic dosage, all thiazides are approximately equal in their diuretic potency. The mechanism whereby thiazides function in the control of hypertension is unknown.
-
Indications and Usage
Saluron® is indicated as adjunctive therapy in edema associated with congestive heart failure, hepatic cirrhosis and corticosteroid and estrogen therapy.
Saluron® has also been found useful in edema due to various forms of renal dysfunction, such as nephrotic syndrome, acute glomerulonephritis, and chronic renal failure.
Saluron® is indicated in the management of hypertension, either as the sole therapeutic agent or to enhance the effectiveness of other antihypertensive drugs in the more severe forms of hypertension.
Usage In Pregnancy
The routine use of diuretics in an otherwise healthy woman is inappropriate and exposes mother and fetus to unnecessary risk. Diuretics do not prevent development of toxemia of pregnancy, and there is no satisfactory evidence that they are useful in the treatment of developed toxemia.
Edema during pregnancy may arise from pathological causes or from the physiologic and mechanical consequences of pregnancy. Thiazides are indicated in pregnancy when edema is due to pathologic causes, just as they are in the absence of pregnancy (however, see Warnings below). Dependent edema in pregnancy, resulting from restriction of venous return by the expanded uterus, is properly treated through elevation of the lower extremities and use of support hose. Use of diuretics to lower intravascular volume in this case is illogical and unnecessary. There is hypervolemia during normal pregnancy which is harmful to neither the fetus nor the mother (in the absence of cardiovascular disease), but which is associated with edema, including generalized edema, in the majority of pregnant women. If this edema produces discomfort, increased recumbency will often provide relief. In rare instances, this edema may cause extreme discomfort which is not relieved by rest. In these cases, a short course of diuretics may provide relief and may be appropriate.
- Contraindications
-
Warnings
Saluron® should be used with caution in severe renal disease. In patients with renal disease, thiazide may precipitate azotemia. Cumulative effects of the drug may develop in patients with impaired renal function.
Thiazides should be used with caution in patients with impaired hepatic function or progressive liver disease, since minor alterations of fluid and electrolyte balance may precipitate hepatic coma.
Thiazides may be additive or potentiative of the action of other antihypertensive drugs. Potentiation occurs with ganglionic or peripheral adrenergic blocking drugs.
Sensitivity reactions may occur in patients with a history of allergy or bronchial asthma.
The possibility of exacerbation or activation of systemic lupus erythematosus has been reported.
-
Precautions
General
All patients receiving thiazide therapy should be observed for clinical signs of fluid or electrolyte imbalance, namely, hyponatremia. hypochloremic alkalosis, and hypokalemia. Serum and urine electrolyte determinations are particularly important when the patient is vomiting excessively or receiving parenteral fluids. Medication such as digitalis may also influence serum electrolytes. Warning signs, irrespective of cause, are: dryness of mouth, thirst, weakness, lethargy, drowsiness, restlessness, muscle pains or cramps, muscular fatigue, hypotension, oliguria, tachycardia, and gastrointestinal disturbances such as nausea and vomiting.
Hypokalemia may develop with thiazides as with any other potent diuretic, especially with brisk diuresis. when severe cirrhosis is present, or during concomitant use of corticosteroids, including ACTH.
Interference with adequate oral electrolyte intake will also contribute to hypokalemia. Digitalis therapy may exaggerate the metabolic effects of hypokalemia, especially with respect to myocardial activity.
Any chloride deficit is generally mild and usually does not require specific treatment, except under extraordinary circumstances (as in liver disease or renal disease). Dilutional hyponatremia may occur in edematous patients in hot weather. Appropriate therapy is water restriction, rather than administration of salt, except in rare instances when the hyponatremia is life-threatening. In actual salt depletion, appropriate replacement is the therapy of choice.
Hyperuricemia may occur or frank gout may be precipitated in certain patients receiving thiazide therapy.
Insulin requirements in diabetic patients may be increased, decreased, or unchanged. Latent diabetes mellitus may become manifested during thiazide administration.
Thiazide drugs may increase the responsiveness to tubocurarine.
The antihypertensive effects of the drug may be enhanced in the postsympathectomy patient.
Thiazides may decrease arterial responsiveness to norepinephrine. This diminution is not sufficient to preclude effectiveness of the pressor agent for therapeutic use.
If progressive renal impairment becomes evident, as indicated by rising nonprotein nitrogen or blood urea nitrogen, a careful reappraisal of therapy is necessary with consideration given to withholding or discontinuing diuretic therapy.
Thiazides may decrease serum PBI levels without signs of thyroid disturbance.
Lithium generally should not be given with diuretics, because they reduce its renal clearance and increase the risk of lithium toxicity. Read circulars for lithium preparations before use of such concomitant therapy with Saluron®.
-
Information for Patients
This medicine may cause a loss of potassium from your body. To help prevent this, your doctor may want you to:
- take another medicine or
- eat or drink foods having high potassium content (such as orange or other citrus fruit juices), or
- take a potassium supplement
It is very important to follow these directions. Also, it is important not to change your diet on your own. This is more important if you are already on a special diet (as for diabetes), or if you are taking a potassium supplement or a medicine to reduce potassium loss. Extra potassium may not be necessary and, in some cases, could be harmful.
Check with your doctor if you become sick and have severe or continuing vomiting or diarrhea. These problems may cause you to lose additional water and potassium.
Caution: Diabetics-Thiazide diuretics may raise blood sugar levels. While you are using this medicine, be especially careful in testing for sugar in your urine. If you have any questions about this, check with your doctor.
A few people who take this medicine may become more sensitive to sunlight than they are normally. When you begin to take this medicine, avoid too much sun or use of a sunlamp until you see how you react, especially if you tend to burn easily. If you have a severe reaction, check with your doctor.
For patients taking this medicine for high blood pressure:
•Do not take other medicines unless they have been discussed with your doctor. This especially includes over-the-counter (nonprescription) medicines for appetite control, asthma, colds, cough, hay fever, or sinus.
Laboratory Tests
Periodic determination of serum electrolytes to detect possible electrolyte imbalance should be performed at appropriate intervals.
Drug Interactions
Anticoagulants (oral): effects may be decreased when used concurrently with thiazide diuretics; dosage adjustments may be necessary.
Antigout medications: thiazide diuretics may raise the level of blood uric acid; dosage adjustment of antigout medications may be necessary to control hyperuricemia and gout.
Other antihypertensive medications, especially diazoxide; pre-anesthetic and anesthetic agents used in surgery; skeletal muscle relaxants, nondepolarizing, used in surgery: effects may be potentiated when used concurrently with thiazide diuretics; dosage adjustments may be necessary.
Amphotericin B or Corticosteroids, including Corticotropin (ACTH): concurrent use with thiazide diuretics may intensify electrolyte imbalance, particularly hypokalemia.
Cardiac glycosides: concurrent use with thiazide diuretics may enhance the possibility of digitalis toxicity associated with hypokalemia.
Colestipol: may inhibit gastrointestinal absorption of the thiazide diuretics; administration 1 hour before or 4 hours after colestipol is recommended.
Hypoglycemics: thiazide diuretics may raise blood glucose levels. For adult-onset diabetics, dosage adjustment of hypoglycemic medications may be necessary during and after thiazide diuretic therapy; insulin requirements may be increased, decreased, or unchanged.
Lithium salts: concurrent use with thiazide diuretics is not recommended, as they may provoke lithium toxicity because of reduced renal clearance.
Methenamine: effectiveness may be decreased when used concurrently with thiazide diuretics, because of alkalinization of the urine.
Diagnostic Interference
With expected physiologic effects.
Blood and urine glucose levels: usually only in patients with a predisposition to glucose intolerance.
Serum bilirubin levels: displacement from albumin binding.
Serum calcium levels: thiazide diuretics should be discontinued before parathyroid function tests are carried out.
Serum uric acid levels: may be increased.
Serum magnesium, potassium, and sodium levels: may be decreased, serum magnesium levels may increase in uremic patients.
Serum protein-bound iodine (PBI) levels: may be decreased.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No long-term studies in animals have been performed to evaluate carcinogenic or mutagenic potential or impairment of fertility.
Pregnancy
Pregnancy Category D. See "WARNINGS"Section.
Teratogenic Effects
Saluron® can cause fetal harm when administered to a pregnant woman. The hazards include fetal or neonatal jaundice, thrombocytopenia, and possibly other adverse reactions which have occurred in adults. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus.
-
Adverse Reactions
The following adverse reactions have been observed, but there has not been enough systematic collection of data to support an estimate of their frequency.
Gastrointestinal system reactions: anorexia, gastric irritation, nausea, vomiting, cramping, diarrhea, constipation, jaundice (intrahepatic cholestatic jaundice), pancreatitis.
Central nervous system reactions: dizziness, vertigo, parathesias, headache, xanthopsia.
Hematologic reactions: leukopenia, agranulocytosis, thrombocytopenia, aplastic anemia.
Dermatologic-Hypersensitivity reactions: purpura, photosensitivity, rash, urticaria, necrotizing angiitis (vasculitis) (cutaneous vasculitis).
Cardiovascular reaction: orthostatic hypotension may occur and may be aggravated by alcohol, barbiturates, or narcotics.
Other: hyperglycemia, glycosuria, hyperuricemia, muscle spasm, weakness, restlessness.
Whenever adverse reactions are moderate or severe, thiazide dosage should be reduced or therapy withdrawn.
-
Overdosage
Signs and Symptoms
Diuresis, lethargy progressing to coma, with minimal cardiorespiratory depression and with or without significant serum electrolyte changes or dehydration; GI irritation; hypermotility; transient elevation of BUN level.
Treatment
Empty stomach by gastric lavage, taking care to avoid aspiration. Monitor serum electrolyte levels and renal function, and institute supportive measures, as required to maintain hydration, electrolyte balance, respiration, and cardiovascular and renal function. Treat GI effects symptomatically.
-
Dosage and Administration
In the treatment of edema, the usual initial dose is 50 to 200 mg daily, in 1 or 2 divided doses, reduced to a dose of 25 to 50 mg on alternate days or intermittently. In the treatment of hypertension, the usual dose is 25 to 50 mg daily in 1 or 2 divided doses, either alone, or in conjunction with other antihypertensive agents. A suggested initial dose for children is 1 mg per kg of body weight daily, reduced for maintenance.
Therapy should be individualized according to patient response. This therapy should be titrated to gain maximal therapeutic response, as well as the minimal dose possible to maintain that therapeutic response.
- How Supplied
- N/A - Section Title Not Found In Database
-
INGREDIENTS AND APPEARANCE
SALURON
hydroflumenthiazide tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:54092-055 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength hydroflumethiazide (UNII: 501CFL162R) (hydroflumethiazide - UNII:501CFL162R) 50 mg Inactive Ingredients Ingredient Name Strength microcrystalline cellulose () lactose () magnesium stearate (UNII: 70097M6I30) colloidal silicon dioxide () sodium starch glycolate () Product Characteristics Color WHITE Score 2 pieces Shape ROUND Size 8mm Flavor Imprint Code RPC;055 Contains Coating false Symbol false Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54092-055-01 100 in 1 BOTTLE Labeler - Shire
