Label: SYNOVEX H- testosterone propionate and estradiol benzoate implant
- NDC Code(s): 54771-3901-1
- Packager: Zoetis Inc.
- Category: OTC ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Animal Drug Application
Drug Label Information
Updated July 6, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONSynovex® H - (testosterone propionate and estradiol benzoate implants) 200 mg testosterone propionate and 20 mg estradiol benzoate per implant - SUBCUTANEOUS IMPLANTS FOR GROWING BEEF HEIFERS FED ...
-
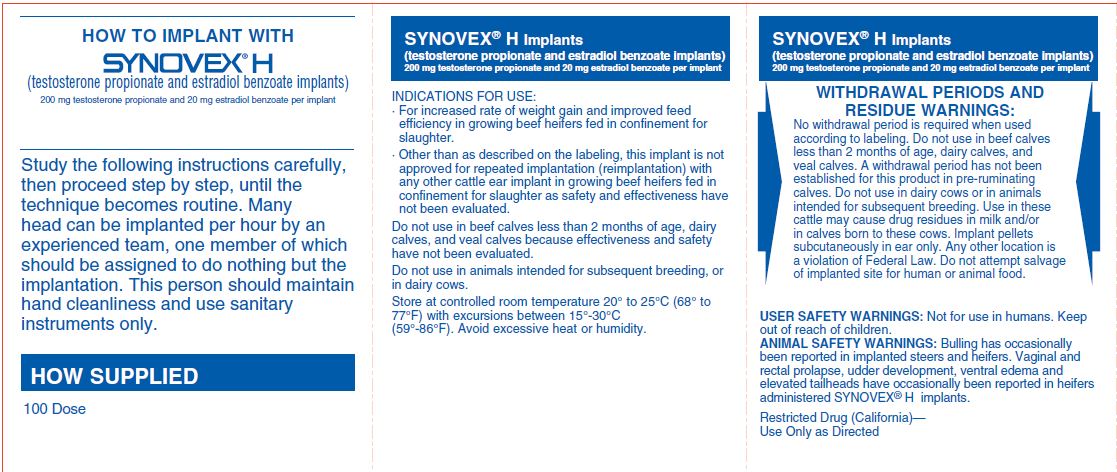
WITHDRAWAL PERIODS AND RESIDUE WARNINGSNo withdrawal period is required when used according to labeling. Do not use in beef calves less than 2 months of age, dairy calves, and veal calves. A withdrawal period has not been established ...
-
USER SAFETY WARNINGSNot for use in humans. Keep out of reach of children.
-
ANIMAL SAFETY WARNINGSBulling has occasionally been reported in implanted steers and heifers. Vaginal and rectal prolapse, udder development, ventral edema and elevated tailheads have occasionally been reported in ...
-
INDICATIONS FOR USE· For increased rate of weight gain and improved feed efficiency in growing beef heifers fed in confinement for slaughter. · Other than as described on the labeling, this implant is not approved ...
-
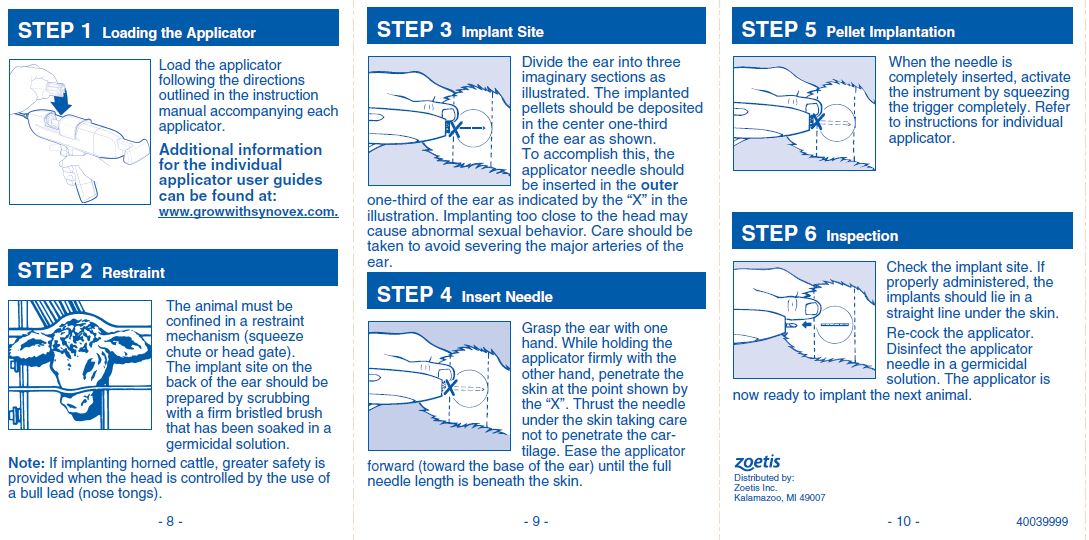
DosageImplant complete contents of one cartridge cell per heifer. Approved implantation technique is fully described in the package insert. NOTE: Never sacrifice careful, clean technique for speed of ...
-
DIRECTIONSImplant complete contents of one cartridge cell per heifer. Approved implantation technique is fully described in the package insert. NOTE: Never sacrifice careful, clean technique for speed of ...
-
StorageStore at controlled room temperature 20°-25°C (68°-77°F) with excursions between 15°-30°C (59°-86°F). Avoid excessive heat or humidity.
-
HOW SUPPLIED100 Dose
-
QUESTIONS/COMMENTS?For a copy of the Safety Data Sheet or to report side effects, contact Zoetis Inc. at 1-888-963-8471. For additional information about reporting side effects for animal drugs, contact FDA at ...
-
SPL UNCLASSIFIED SECTIONDistributed by: Zoetis Inc. Kalamazoo, MI 49007 - Revised: May 2023 - 40039999
-
PRINCIPAL DISPLAY PANEL - Carton of 10 cartridges40039999
-
INGREDIENTS AND APPEARANCEProduct Information