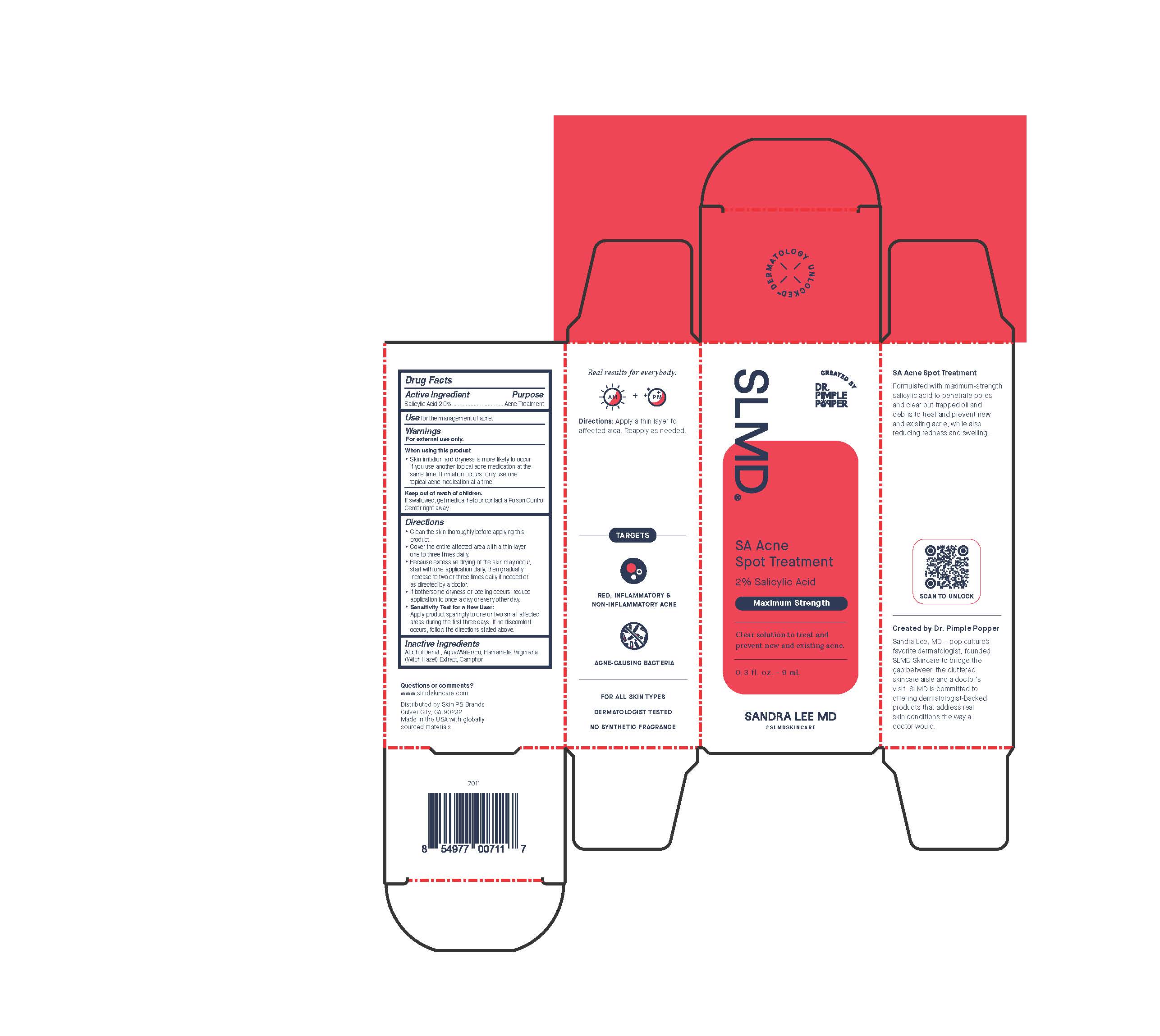
Label: SA ACNE SPOT TREATMENT- acne spot treatment lotion
- NDC Code(s): 73318-7003-9
- Packager: Skin PS Brands
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 13, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Use
- Warnings
- When using this product
- Keep out of reach of children.
-
Directions
- clean skin thoroughly before applying this product
- cover the entire affected area with a thin layer one to three times daily
- because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three daily if needed or as directed by a doctor
- if bothersome dryness or peeling occurs, reduce application to once a day or every other day
- Sensitivity Test for a New User:
Apply product sparingly to one or two small affected areas during the first 3 days. if no discomfort occurs, follow the directions stated above.
- Inactive Ingredients
- Questions or comments?
- Real results for everybody.
-
SA Acne Spot Treatment
Formulated with maximum-strength salicylic acid to penetrate pores and clear out trapped oil and debris to treat and prevent new and existing acne, while also reducing redness and swelling.
Created by Dr. Pimple Popper
Sandra Lee, MD - pop culture's favorite dermatologist, founded SLMD Skincare to bridge the gap between the cluttered skincare aisle and a doctor's visit. SLMD is committed to offering dermatologist-backed products that address real skin conditions the way a doctor would.
- SLMD®
-
INGREDIENTS AND APPEARANCE
SA ACNE SPOT TREATMENT
acne spot treatment lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73318-7003 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 20 mg in 1 g Inactive Ingredients Ingredient Name Strength HAMAMELIS VIRGINIANA BARK (UNII: IH3063S9MY) CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73318-7003-9 1 in 1 CARTON 07/05/2023 1 9 g in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 07/05/2023 Labeler - Skin PS Brands (081085221) Registrant - Westwood Laboratories, LLC. (832280635) Establishment Name Address ID/FEI Business Operations Westwood Laboratories, LLC. 832280635 manufacture(73318-7003) , label(73318-7003) , pack(73318-7003)